A. Salehipour1,3, M. Dolatshahi2,3, M. Haghshomar3,4, J. Amin5
1. Neurosurgery Research Group (NRG), Student Research Committee, Hamadan University of Medical Sciences, Hamadan, Iran; 2. Mallinckrodt Institute of Radiology, Division of Neuroradiology, Washington University in St. Louis, St. Louis, MO, USA; 3. NeuroImaging Network (NIN), Universal Scientific Education and Research Network (USERN), Tehran, Iran; 4. Students’ Scientific Research Center (SSRC), Tehran University of Medical Sciences, Tehran, Iran; 5. Clinical Neurosciences, Clinical and Experimental Sciences, Faculty of Medicine, University of Southampton, Southampton, UK.
Corresponding Author: Dr. Jay Amin (BM.PhD), Associate Professor in Psychiatry of Older Age, University of Southampton, Clinical Neurosciences, Clinical and Experimental Sciences, Faculty of Medicine, University of Southampton, United Kingdom, Phone: 02380475206, Email: jay.amin@soton.ac.uk
J Prev Alz Dis 2023;2(10):276-286
Published online February 20, 2023, http://dx.doi.org/10.14283/jpad.2023.20
Abstract
Imbalances in thyroid hormones have been linked with Alzheimer’s dementia. Several studies have reported an association between thyroid disorders, such as hyper- or hypothyroidism, with Alzheimer’s disease. However, there remains no consensus about the precise role of thyroid dysfunction in Alzheimer’s disease. In this study we systematically searched PubMed, Embase and Scopus for clinical studies which reported the prevalence of hyper- or hypothyroidism in people with Alzheimer’s disease compared to controls. Meta-analysis was performed to compare thyroid disorder prevalence in Alzheimer’s disease and controls. Subgroup analysis was performed to assess the clinical and subclinical thyroid dysfunction, separately. Seven studies, including 1189 people with Alzheimer’s disease and 72711 controls, were included in our sample. Hypothyroidism was significantly more prevalent in Alzheimer’s disease compared with controls (6.4% vs 2.4%, p=0.01). Subgroup analysis showed that clinical hypothyroidism was not significantly different between Alzheimer’s disease compared to controls (10.0% vs 5.3%, p=0.35). There was no difference in the crude overall prevalence of clinical and subclinical hyperthyroidism in Alzheimer’s disease versus controls (2.4 vs 1.9%, p=0.73). Our analyses revealed a higher prevalence of hypothyroidism in Alzheimer’s disease. Whether this finding is explained by hypothyroidism being a risk factor for, or consequence of, Alzheimer’s disease requires longitudinal analysis. Our review supports further work into a potential role for treatment of hypothyroidism in the prevention or delay of Alzheimer’s disease.
Key words: Alzheimer’s disease, thyroid, hypothyroidism, hyperthyroidism, meta-analysis.
Introduction
Worldwide, Alzheimer’s disease (AD) cases are expected to reach 152.8 million in 2050 (1). Despite this, our understanding of the factors relating to the etiology and progression of AD remains limited, and there are no disease modifying treatments for this most common cause of dementia (2). The prevalence of hypothyroidism in the general population is thought to be 5% and an additional 5% of people are thought to go undiagnosed (3). The prevalence of hyperthyroidism is around 1% in the general population (4). This systematic review will seek to explore the relationship between AD and thyroid dysfunction, both common disorders of older age. By doing this we will discuss potential underlying biological mechanisms that may explain any association found.
Thyroid hormones include thyroxine (T4) and the biologically more active triiodothyronine (T3). The secretion of these hormones is regulated by thyroid stimulating hormone (TSH) and by extension, thyroid releasing hormone (TRH). Thyroid hormones have receptors in most human tissues, rendering them highly influential on a myriad of processes in metabolism and homeostasis. They affect brain metabolism, neurogenesis, myelination, and cellular repair throughout life (5). Transthyretin transports circulating T4 and T3 to the central nervous system (6). Thyroid hormone receptors have been found in human neuronal cells from the tenth week of pregnancy, supporting their role in neurodevelopment (7). Additionally, basal forebrain and hippocampal cholinergic function are particularly affected by thyroid hormones (8). Thyroid hormones may therefore be considered neuro-regulators of the central nervous system.
Thyroid hormone disturbances contribute to various developmental, metabolic, and age-related disorders, and may be associated with dementia (9) and cognitive impairment (10). Abnormal thyroid hormone signaling has been reported in pre-clinical studies to increase β-amyloid (Aβ) levels and also tau phosphorylation (11-13), both of which are hallmarks of AD pathology (14), suggesting a pathobiological mechanism linking thyroid dysfunction with AD. Thyroid function also affects glucose metabolism, alterations of which have been associated with AD pathology (15, 16).
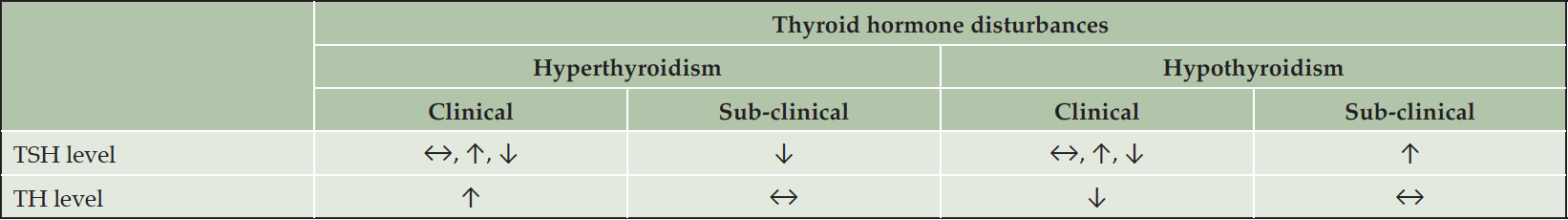
Accordingly, it can be hypothesized that disturbances in thyroid hormone levels may be associated with AD. Thyroid dysfunction, namely hyper- and hypothyroidism, have distinct clinical characteristics. Common symptoms include, fatigue, tremor, anxiety, disturbed sleep, weight loss and heat intolerance for hyperthyroidism, and fatigue, lethargy, cold intolerance, dry skin, and unexplained weight gain for hypothyroidism (4, 17). Hypothyroidism can also cause an enlarged thyroid, high cholesterol, brittle nails, constipation, irritability, sexual dysfunction, bradycardia, or irregular uterine bleeding (18). Of note, an atypical form of hyperthyroidism, apathetic thyrotoxicosis, is characterized by lethargy and can be seen in the elderly. Despite having hyperthyroidism, these patients are not anxious or restless, but instead present as withdrawn, apathetic, or confused (19, 20). Both hyper- and hypothyroidism can be classified as clinical or subclinical, depending on the specific thyroid hormone and TSH levels (21). Subclinical hyper-/hypothyroidism is defined when TSH levels are lower/higher than the upper normal reference range while peripheral thyroid hormones remain within the normal reference ranges (22, 23). Clinical hyper-/hypothyroidism, however, is diagnosed when peripheral thyroid hormones are abnormally high or low, respectively, which has numerous causes and classifications (17, 21). The profile of thyroid hormone changes in clinical and sub-clinical hyper- and hypothyroidism is summarized in Table 1.
TH, thyroid hormones; TSH, thyroid stimulating hormone; ↔, within normal reference range; ↑, higher than normal reference range; ↓, lower than normal reference range
Multiple studies have reported an association between an increased risk of dementia or AD with either high (24-30) or low thyroid hormone levels (31-34), with many studies reporting associations with subclinical or clinical hyper-/hypothyroidism with AD (28, 30, 32, 33, 35). More specifically, a prospective longitudinal study assessing if abnormal thyroid hormone levels can predict dementia, found that higher free T4 (fT4) levels were predictive of new-onset dementia in older men, independent of conventional risk factors for cognitive decline (24). In a community-based study of 194 individuals higher TSH was associated with a diagnosis of dementia (31). Conversely, in a cross-sectional study, AD patients have been found to have significantly lower TSH levels compared to controls independent of other risk factors, with lower TSH being linked to a more than two-fold increase in risk of AD (35). Also, greater fT4 and T3 levels have been linked to hippocampal and amygdala atrophy, indicating that hyperthyroidism may play a part in the onset of AD (36).
Whether hyper- or hypothyroidism is directly linked with AD remains unclear (37-39). Despite a number of studies having reported an association (28, 35, 40) or lack thereof (36, 41-43) between hyper-/hypothyroidism (28, 30, 33), or specific thyroid hormone levels (36, 40, 44, 45) and AD, there is no clear conclusion regarding the precise AD-thyroid relationship. We therefore aim to examine the relationship between AD and clinical or subclinical thyroid dysfunction. We hope to discover how AD is associated with hyperthyroidism and hypothyroidism, separately, and whether clinical and/or subclinical thyroid dysfunction plays a role. We have, therefore, conducted a robust meta-analysis of studies reporting the prevalence of hyper- or hypothyroidism in patients with AD with a view to defining how AD is associated with these two thyroid disorders.
Methods
This systematic review and meta-analysis was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline (46) and was registered in the Prospective Register of Systematic Reviews (PROSPERO) website (https://www.crd.york.ac.uk/prospero/) with the following ID: CRD42020078556. This study was reviewed and approved by the Tehran University of Medical Sciences ethics committee (reference number: IR.TUMS.VCR.REC.1398.559).
Literature Search and Selection Criteria
We searched PubMed (MEDLINE), Embase, and Scopus on 10th November 2021. The search terms were ((AD OR Alzheimer’s disease OR Alzheimer disease OR amyloid-beta OR beta-amyloid) AND (Thyroxine OR T4 OR Triiodothyronine OR T3 OR Thyrotropin OR TSH OR hypothyroidism OR hyperthyroidism OR thyrotoxicosis OR grave’s disease OR Hashimoto’s)). We have provided the search string for each database in the supplementary materials. Only English articles were included, with no limitation on publication dates. In addition, all references that were cited within the identified papers were screened for inclusion. Covidence.org was used to store search results, identify duplicates, and track screening decisions. Screening of search results was performed by two registered medical practitioner reviewers using pre-specified inclusion and exclusion criteria. In case of disagreement about whether a study should be included or not, a third reviewer decided on study inclusion or exclusion.
Original studies were included if they met the following criteria: (1) studies with an observational design providing blood concentrations of thyroid hormones in an AD population, or the number of AD subjects reported to have clinical/subclinical hyper- or hypothyroidism based on any criteria used by the study, comparing them to control subjects; and (2) articles providing the total number of subjects in both AD and control groups.
Studies were excluded if: (1) they were reviews, letters, case reports, or commentaries; (2) interventional studies which did not report thyroid hormone concentrations in the absence of intervention; (3) animal, or in vitro studies; (4) study populations with comorbidities such as Down syndrome; (5) studies including patients with non-AD dementias, which did not report AD data separately; (6) studies comparing AD to subjects other than controls; and (7) studies with no retrievable full-texts. We contacted the authors of studies where the full text was not available and whose email we could attain (14 Authors), but were not successful in obtaining the full text.
Of note, no specific criteria for diagnosis of AD or hyper-/hypothyroidism were considered in this meta-analysis for the selection process, and any criteria used by the selected papers were accepted. In addition, as long as a paper explicitly reported the number of subjects diagnosed with AD and hyper-/hypothyroidism, but failed to report on what basis these diagnoses were made, they would nonetheless be included in our analysis.
Data Extraction
To ensure the reliability of the extracted data, two reviewers (A.S. and M.D.) independently reviewed each full-text article, determined whether the study met inclusion/exclusion criteria, and extracted the data to a Microsoft Excel database. In addition to the number of people in the control and AD groups with hyper-/hypothyroidism, further data were extracted including study characteristics i.e., lead author, publication year, participant characteristics i.e., age, sex and mini-mental state examination (MMSE) scores, the specific criteria used for diagnosing hyper-/ hypothyroidism, and reported underlying etiology for hyper-/hypothyroidism.
Quality Assessments
The quality of studies included was independently assessed by two authors (M.H. and A.S.) using the Newcastle-Ottawa Scale (NOS), which is designed for observational studies. In case of disagreement about study quality, a third author (M.D.) decided on the scores. The NOS scale evaluates the main components of observational studies, including sample selection, comparability of cases and controls, and exposure (47). Using this scale, the scores for each study can vary from 0 to 9. Studies with a score of 7 to 9 have the lowest risk of bias and the highest quality, while studies with a score of less than 4 have the highest risk of bias and the lowest quality. Studies with a score of 4 to 6 have a moderate risk of bias (47). Studies with a high risk of bias were excluded.
Data Synthesis and Meta-analysis
Data analysis was performed using Cochrane Review Manager 5.4.1. Odds ratio was used as the effect size measure for comparing the prevalence of hyper- or hypothyroidism between AD patients and controls. For sensitivity analysis, we performed meta-analyses in subclinical and clinical subgroups, separately. Based on Cochrane guidelines, Q statistic tests and the I2 index were used for the assessment of heterogeneity (48, 49). I2 index estimates the percentage of the total observed variation in the studies, which is attributable to heterogeneity rather than chance, where an I2 value of more than 50% is predictive of significant heterogeneity among studies. Forest plots were used for presenting results of the meta-analysis and the heterogeneity among the studies (48, 49). Unless there was significant heterogeneity, we used a fixed-effects model for meta-analyses; otherwise, a random-effects model was applied. A P-value of < 0.1 for Cochran’s Q test of heterogeneity and a P-value of < 0.05 for other analyses were considered statistically significant.
Results
Search outcomes
In total, 3585 records were identified. After removing duplicates, 1727 records were screened for title and abstract, out of which 1389 papers were excluded only based on their titles and abstracts without full text assessment. At the next stage 338 papers were selected for full text assessment out of which 17 could not be retrieved, and so 321 full-text articles were assessed for eligibility. After exclusions, seven studies, including 1189 AD subjects and 72711 controls, were included in this systematic review and meta-analyses. The PRISMA (46) diagram shows detailed information on the number of ineligible studies and the reasons for exclusion (Figure 1). No studies were excluded for a high risk of bias.
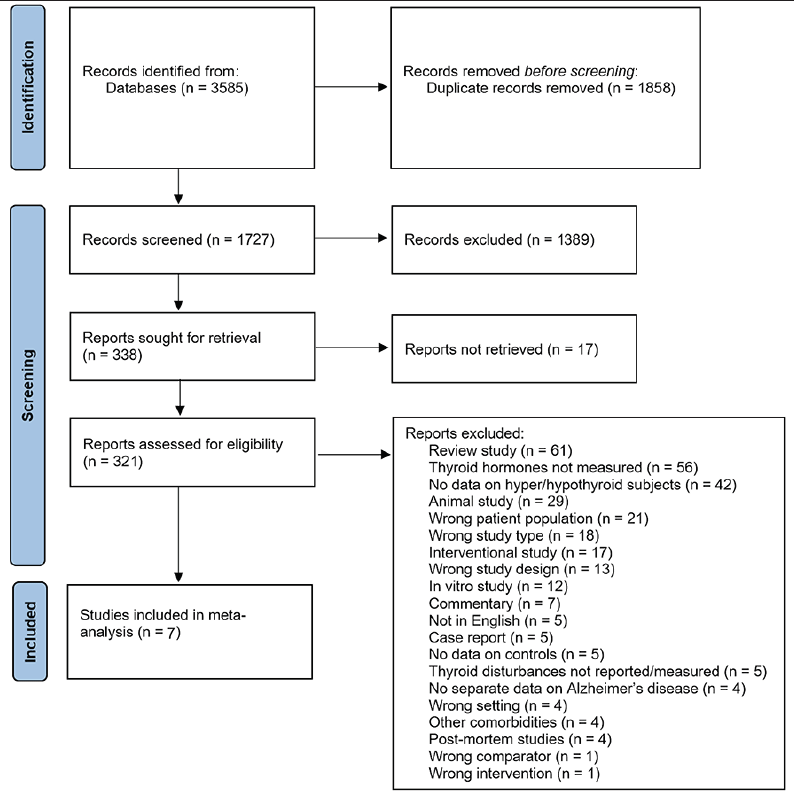
Figure 1. The Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) screening flow-chart
At the first stage of screening 1389 papers were excluded based on their titles and abstracts without full text assessment. At the next stage 338 papers were selected for full text assessment out of which 17 could not be retrieved.
Characteristics of included studies
The seven studies included in this systematic review and meta-analysis were conducted in the United states (50, 51), Mexico (52), Cuba (53), United Kingdom (54), China (55), and Russia (56). The earliest study was performed by Small et al. in 1985 (50) and the most recent study was conducted by Ranzola et al. in 2019 (53). The included studies employed a case-control or cross-sectional study design and the sample size varied from 38 (51) to 72,878 (54) (Table 2, 3). Almost all of the included studies used different diagnosis criteria for AD (Table 2, 3). Four studies stated the method by which they diagnosed hyper-/ hypothyroidism (Table 2, 3), using either radioimmunoassay, chemiluminescent, or electrochemiluminescent immunoassay techniques to measure thyroid hormone levels.
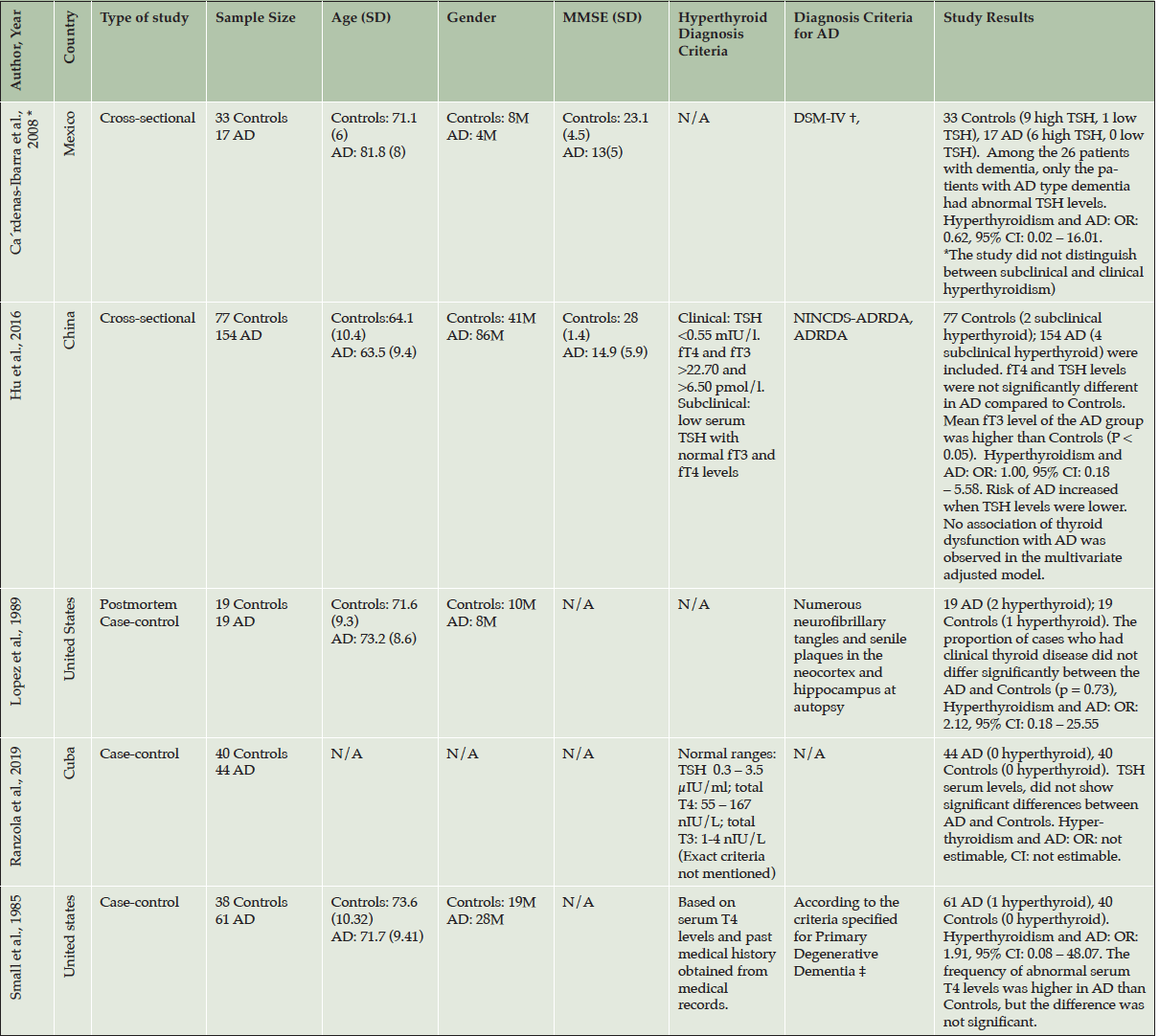
Table 2. Study characteristics of the included papers in the analysis reporting on hyperthyroidism and AD
AD: Alzheimer’s disease; TSH: Thyroid Stimulating Hormone; fT4: free T4; fT3: free T3; C: clinical; S: Subclinical; N/A: Not Available; OR: Odds Ratio; CI: Confidence Interval; ADNC, Operationalization of AD neuropathologic changes; CERAD, Consortium to Establish a Registry for Alzheimer’s Disease; ICD-10, International Classification of Disease, 10th edition; NINCDS, National Institute of Neurological and Communicative Disorders; ADNRDA, Alzheimer’s Disease and Related Disorders Association; *: This study had two different analysis groups, only the RSS group fitted our criteria; †: cognitive MMSE score , GDS score , and the HIS; ‡: Based on Diagnostic and Statistical Manual of Mental Disorders issued by the American Psychiatric Association
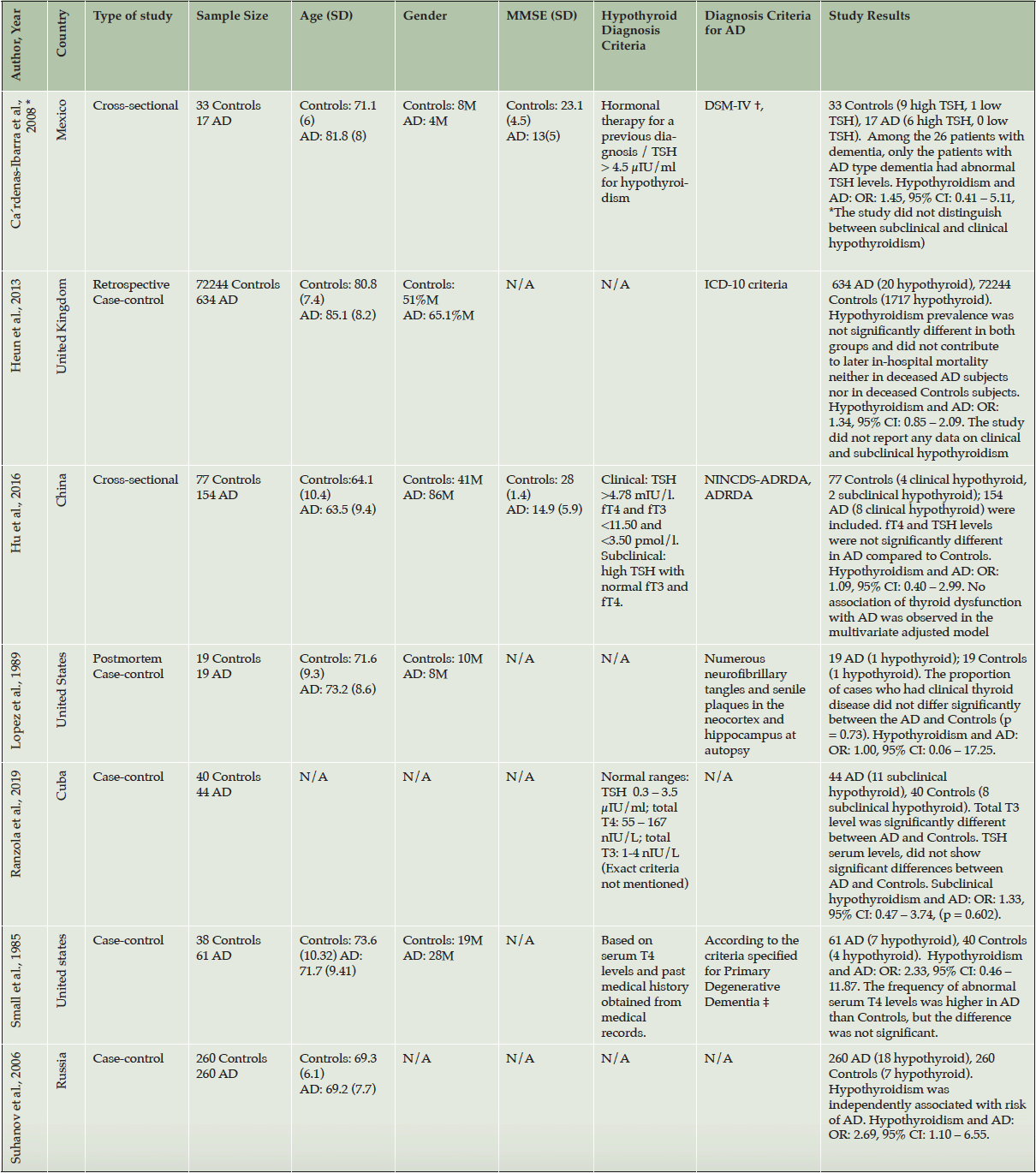
Table 3. Study characteristics of the included papers in the analysis reporting on hypothyroidism and AD
AD: Alzheimer’s disease; TSH: Thyroid Stimulating Hormone; fT4: free T4; fT3: free T3; C: clinical; S: Subclinical; N/A: Not Available; OR: Odds Ratio; CI: Confidence Interval; ADNC, Operationalization of AD neuropathologic changes; CERAD, Consortium to Establish a Registry for Alzheimer’s Disease; ICD-10, International Classification of Disease, 10th edition; NINCDS, National Institute of Neurological and Communicative Disorders; ADNRDA, Alzheimer’s Disease and Related Disorders Association; *: This study had two different analysis groups, only the RSS group fitted our criteria; †: cognitive MMSE score , GDS score , and the HIS; ‡: Based on Diagnostic and Statistical Manual of Mental Disorders issued by the American Psychiatric Association
Out of the seven included studies, five studies (50-53, 55) reported rates of both hyperthyroidism and hypothyroidism, and two studies (42, 54, 57) presented data only on hypothyroidism. Two studies (52, 55) assessed thyroid dysfunction in different types of dementia and did not focus only on AD, and two studies (54, 56) reported hyper-/ hypothyroidism only as co-morbidities or risk factors amongst a range of other conditions. None of the studies reported on the underlying etiology for hyper-/hypothyroidism.
Quality Assessment & Risk of Bias
The mean NOS score for the included studies was 7.75 (± SD 1.28, range: 6 – 9), which indicated good quality overall. Two studies had moderate (4 – 6) risk of bias and the other five studies had low (7 – 9) risk of bias (Supplementary table 1).
Publication bias
The occurrence of publication bias was evaluated using funnel plots (Supplementary figure 1). Regarding hypothyroidism, the funnel plot was minimally asymmetric around the cumulative effect size and therefore did not suggest publication bias. Regarding hyperthyroidism, the funnel plot showed symmetrical distribution and therefore also did not suggest of publication bias. The Egger and Begg regression tests were not performed due to the limited number of studies.
AD, Alzheimer’s disease
Hypothyroidism and Alzheimer disease
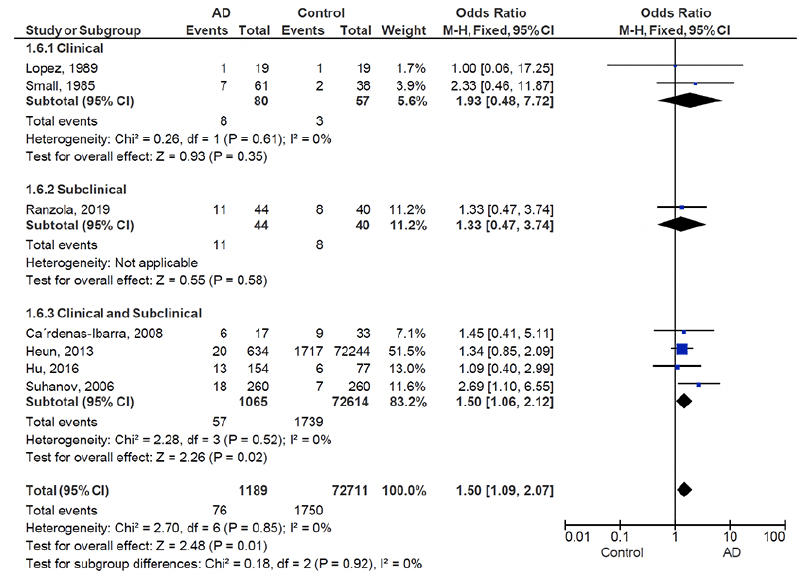
Five of the seven studies included in this paper did not report an association between thyroid disease and AD and only two reported a significant difference between AD and controls in terms of prevalence of hypothyroidism (52, 56). Of these two studies, Suhanov et al. reported hypothyroidism as an independent risk factor associated with AD (56). The estimated overall prevalence of hypothyroidism in patients with AD and controls is presented as a forest plot (Figure 2). The crude overall prevalence of clinical and subclinical hypothyroidism was 6.4% (76 of 1189) in the pooled sample of AD patients and 2.4% (1750 of 72711) in the pooled sample of controls, with a significant between group difference (odds ratio (OR):1.50, 95% confidence interval (CI): 1.09 – 2.07, z = 2.48, p = 0.01; test of heterogeneity: I2 = 0%, p = 0.85, Figure 2). Considering the subgroups separately, the crude overall prevalence of clinical hypothyroidism was 10% (8 of 80) in AD patients, and 5.3% (3 of 57) in controls demonstrating a non-significant difference between AD and control groups (OR: 1.93, 95% CI: 0.48 – 7.72, z = 0.93, p = 0.35; test of heterogeneity: I2 = 0% p = 0.61, Figure 2). The single study assessing subclinical hypothyroidism showed a crude overall prevalence of 25% (11 of 44) in AD patients, and 20% (8 of 40) of controls with no significant difference between ADs and controls (OR: 1.33, 95% CI: 0.47 – 3.74, z = 0.55, p = 0.58, Figure 2). Four studies consisted of a mixed clinical and subclinical hypothyroid sample. The crude overall prevalence of hypothyroidism in these studies was 5.3% (57 of 1065) in AD patients, and 2.3% (1739 of 72,614) in controls, which was significantly higher in AD patients (OR: 1.50, 95% CI: 1.06 – 2.12, z = 2.26, p = 0.02; test of heterogeneity: I2 = 0%, p = 0.52, Figure 2). Subgroups based on clinical/subclinical hypothyroidism were not significantly different (I2 = 0%, p = 0.92).
Hyperthyroidism and Alzheimer disease
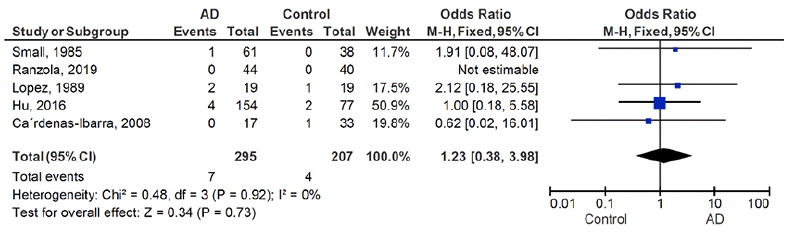
Among studies that reported on hyperthyroidism, none found a significant association between hyperthyroidism and the risk of AD. A total of five studies were included in the meta-analysis. The estimated overall prevalence of hyperthyroidism in patients with AD and controls is presented in a forest plot (Figure 3). The crude overall prevalence of clinical and subclinical hyperthyroidism was 2.3% (7 of 295) AD patients, and 1.9% (4 of 207) in controls (OR: 1.23, 95% CI: 0.38 – 3.98, z=0.34, p=0.73; test of heterogeneity: I2= 0%, p= 0.92, Figure 3), which did not show a significant difference between ADs and controls.
AD: Alzheimer’s disease
Discussion
The precise relationship between thyroid dysfunction and AD has not been previously explored by meta-analysis. In this study, we systematically reviewed studies reporting thyroid dysfunction in people with AD and assessed the association of hyper-/ hypothyroidism with AD by performing a meta-analysis. Our results revealed significantly higher prevalence of hypothyroidism in AD compared to controls, while no difference was observed in the prevalence of hyperthyroidism between the two groups. Analyses of subclinical, clinical, and mixed clinical/subclinical subgroups revealed no significant difference in AD-associated prevalence of hypothyroid patients. Although it is not possible to infer a causative relationship between hypothyroidism and AD from this study, we will explore potential mechanisms behind this association.
Thyroid hormones play an important role in neurogenesis, neurodifferentiation, and normal brain function, particularly learning and memory, with hippocampal neurons being specifically affected by thyroid hormone levels (57-59). Thyroid gland removal has been shown to cause hippocampus-dependent spatial learning and memory impairment in rats (60, 61). Thyroid dysfunction has been considered both as a possible causative factor or a consequence of AD. Interestingly, thyroid dysfunction has been linked to increased risk of AD with studies reporting that genetically predicted levels of thyroid stimulating hormone (TSH), if increased within the normal limits, is associated with a lower risk of AD (62, 63). Also, hypothyroidism causes endoplasmic reticulum dysfunction in the hippocampus and results in higher levels of reactive oxygen species and thus oxidative stress, mechanisms involved in the pathophysiology of AD (61). The theory of thyroid dysfunction causing AD is supported by several studies linking thyroid hormones and amyloid precursor proteins (13, 64).
Thyroid hormones can also affect glucose metabolism and insulin signaling, with studies reporting the association between thyroid dysfunction and altered glucose metabolism (16, 65 66). This association exists between thyroid dysfunction and both type 1 and type 2 diabetes, and with a shared autoimmune pathology, type 1 diabetes and hypothyroidism have been linked more strongly (16). Using positron emission tomography (PET) imaging, brain glucose consumption has been observed to correlate with cognition, specifically in the hippocampus, amygdala, and prefrontal cortex (67). Insulin regulates some important functions in the brain, including cognition and memory (68, 69). There are important mechanisms involved in AD pathology that are affected by insulin, namely energy metabolism, autophagia, oxidative stress, synaptic plasticity, and cognitive functions (70, 71). Whether insulin imbalance causes thyroid dysfunction or vice versa, the literature points to a relatively strong association between the two, however, there needs to be more studies specifically designed to answer whether insulin imbalance is a link between thyroid dysfunction and AD.
Other studies consider thyroid dysfunction to be a consequence of AD rather than causing it. One hypothesis is that neurodegeneration in AD could influence thyroid function, for example a decrease in TRH and TSH could result from adenohypophysis degeneration (37). Supporting this theory is the circadian dysregulation seen in AD patients, which could be explained by adenohypophysis degeneration and its non-responsiveness to stimulants such as TRH, T4, and melatonin (72). In fact, several neuroendocrine dysfunctions are reported in AD including, hypothalamic-pituitary-adrenal axis (73, 74), hypothalamic-pituitary-gonadal axis (74), and hypothalamic-pituitary-thyroid axis (75), all reported to accelerate cognitive decline (74, 76). Early Aβ accumulation possibly contributes to the dysregulation of these axes (77). Further research examining the relationship between adenohypophysis degeneration and AD is warranted.
Previously in studies of animal models, it was demonstrated that the expression of amyloid precursor proteins is negatively regulated by T3 (13). Low levels of T3 limit hippocampal growth, decrease the size of neurons in the brain cortex, reduce the dentate gyrus cell counts, lower myelination, and slow down dendritic and axonal spine formation (78, 79). Neurotransmitter expression, their composing molecules, and their release are also affected by T3. Moreover, it has been shown that supplementation of thyroid hormones in an animal model of hypothyroidism, act against neuroinflammation and formation of amyloid-beta plaques which are known AD-related pathologies (80). Peripheral free T3 and free T4 hormones are positively associated with serum total tau levels, and serum total tau levels are higher in patients with hyperthyroidism. With tau pathology being a hallmark of AD, this shows a possible role of thyroid hormones in AD (81). Furthermore, it has been shown that thyroid hormone receptor alpha gene polymorphisms are associated with risk of AD (82). In patients with systemic amyloidosis, which like AD has a pathology of protein misfolding and aggregation (83), amyloid infiltration may lead to hypothyroidism (85). However, to the best of our knowledge, amyloid and tau pathology have not been reported to directly cause thyroid disease.
Treatments with thyroid hormones have shown promise in preventing the onset and progression of AD in pre-clinical studies. For instance, T4 administration improved cognitive impairment in thyroidectomized rats and attenuated neurodegeneration in diabetic rats via insulin signaling (60, 85). Treatment with T4 has also been shown to reduce hippocampal-dependent memory impairment, neuroinflammation, and Aβ deposition in hypothyroid rats (86). While most studies in the literature that link thyroid dysfunction with AD report hypothyroidism as an associative factor, subclinical hyperthyroidism has also been reported to increase the risk of AD (28, 35, 37, 44), and a decreased TSH level has been associated with an increased risk of AD by Annerbo et al. (30). Some studies have even suggested treating subclinical hypothyroidism when there is evidence of cognitive decline (52, 87, 88). However, thyroid hormone therapy for older adults with subclinical hypothyroidism has not been shown to be beneficial and may even be harmful (89).
The studies which found an association between AD and subclinical hyperthyroidism or lower TSH levels, did not satisfy our inclusion criteria as they did not have adequate data for inclusion in meta-analysis. Indeed, none of the studies included in our sample found an association between clinical or subclinical hyperthyroidism and AD, a fact strengthened by our meta-analysis which similarly found no association. A fairly large cross-sectional study of 1276 individuals found an association between subclinical hyperthyroidism and all-cause dementia in both men and women but an association between subclinical hyperthyroidism and AD was only reported in men (90). Furthermore, no association between subclinical hyperthyroidism and AD was found in the Framingham Heart Study (37). Studies reporting an association between hyperthyroidism and AD suggest there may be a pathological link between the two conditions. One argument posits increased oxidative stress as a result of higher levels of thyroid hormones (91), another points to neuron death caused by increased thyroid function (92). There is another theory linking lower levels of TSH and TRH to decreased levels of acetylcholine (TRH stimulates acetylcholine synthesis and release) (93, 94). Further research is required into the potential mechanisms between hyperthyroidism and AD, but we conclude from our systematic review that on a population basis there is no association between these two conditions.
There were some limitations to our meta-analysis. First, the number of studies assessing the association between hyper-/ hypothyroidism and AD was almost twice as those which met criteria or had the adequate data for inclusion in this study, leading to a relatively low number of included studies in our meta-analysis. Second, almost all studies applied different diagnostic criteria for AD and thyroid dysfunction, and several studies did not report the precise diagnostic criteria that they used. While most of the included studies reported the AD diagnosis criteria, the varied criteria used between studies may result in a lack of consistency in the population examined. Third, none of the papers included reported the precise method used for thyroid hormone measurement. Indeed, some studies also failed to report the criteria by which thyroid dysfunction was defined, and the number of subclinical and/or clinical hyper-/ hypothyroid patients in each group (51, 54, 56). Use of medication that could affect thyroid function by patients in some of the studies (50, 51) is also a factor that may have confounded the results. Fourth, the lack of a unified reference range for TSH and thyroid hormones may cause unreliable results in the diagnosis of hyper-/hypothyroidism. TSH levels can be affected by age, sex and ethnicity. Females have been reported to have higher TSH and fT4 compared to males, and TSH has been reported to rise progressively with older age (95-100). Some of the included studies did not report age or sex and none reported ethnicity separately for AD and controls. We suggest that future studies take this into account to allow for subgroup meta-analyses to find possible associations within subgroups of different age group, sex, and ethnicity. Fifth, the cross-sectional design in most of included studies meant that they are unable to demonstrate the temporality of thyroid dysfunction and AD progression, meaning that it remains uncertain whether thyroid dysfunction precedes AD or occurs as a consequence. This is important as any possible therapeutic or preventive approach considering thyroid dysfunction would be built on the nature of the relationship between thyroid dysfunction and AD. Sixth, the sample sizes of the included studies were unbalanced, with a small sample size in most studies compared to one study conducted by Heun et al. which had a particularly large sample size for controls that has resulted in this study having the largest weight in our meta-analysis. This fact could have a skewing effect on the pooled data. It is worth mentioning that this study did not report diagnosis criteria for thyroid dysfunction. Lastly, publication bias was prominent when assessing studies reporting hypothyroidism.
Conclusion
Our meta-analyses showed that there is an association between hypothyroidism and AD, with hypothyroidism more prevalent in AD compared to controls. This is an important finding as the association between AD and hypothyroidism is now supported by a comprehensive statistical analysis of the available and relevant literature. We support future work in this area, particularly longitudinal studies with an adequate follow-up time, specifically to address some the limitations faced in this study. Limitations including lack of a unified criteria for diagnosis of thyroid dysfunction and AD, unaccounted for differences in sample populations, and other methodological issues – many of which are avoidable. Importantly, our review of the available literature also shows no evidence of an association between hyperthyroidism and AD. It appears that a consensus is building towards hypothyroidism, rather than hyperthyroidism, having an important relationship with AD.
Further studies are needed to establish potential causation rather than association between thyroid dysfunction and AD. We support the examination of whether using thyroid medication to treat clinical and subclinical hypothyroidism could be a potential adjunctive agent in AD treatment. Presented in this study is the first meta-analysis conducted which focuses on thyroid dysfunction among AD patients compared with controls, and marks an important step towards understanding the factors that may influence the development and progression of AD.
Funding: No funding was provided for this study. For the purpose of open access, the author has applied a CC BY public copyright licence to any Author Accepted Manuscript version arising from this submission.
Ethical standards: This systematic review and meta-analysis was registered in the Prospective Register of Systematic Reviews (PROSPERO) website (https://www.crd.york.ac.uk/prospero/) with the following ID: CRD42020078556. This study was reviewed and approved by the Tehran University of Medical Sciences ethics committee (reference number: IR.TUMS.VCR.REC.1398.559).
Conflict of interest: The authors declare no conflicts of interests.
Open Access: This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
References
1. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the Global Burden of Disease Study 2019. The Lancet Public health. 2022;7(2):e105-e125. http://dx.doi.org/10.1016/s2468-2667(21)00249-8.
2. Salehipour A, Bagheri M, Sabahi M, Dolatshahi M, Boche D. Combination Therapy in Alzheimer’s Disease: Is It Time? Journal of Alzheimer’s Disease. 2022;Preprint:1-17. http://dx.doi.org/10.3233/JAD-215680.
3. Chiovato L, Magri F, Carlé A. Hypothyroidism in Context: Where We’ve Been and Where We’re Going. Advances in therapy. 2019;36(Suppl 2):47-58. http://dx.doi.org/10.1007/s12325-019-01080-8.
4. De Leo S, Lee SY, Braverman LE. Hyperthyroidism. Lancet (London, England). 2016;388(10047):906-918. http://dx.doi.org/10.1016/S0140-6736(16)00278-6.
5. Calzà L, Fernández M, Giardino L. Role of the Thyroid System in Myelination and Neural Connectivity. Comprehensive Physiology. 2015;5(3):1405-1421. http://dx.doi.org/10.1002/cphy.c140035.
6. Richardson SJ, Wijayagunaratne RC, D’Souza DG, Darras VM, Van Herck SL. Transport of thyroid hormones via the choroid plexus into the brain: the roles of transthyretin and thyroid hormone transmembrane transporters. Front Neurosci. 2015;9:66. http://dx.doi.org/10.3389/fnins.2015.00066.
7. Bernal J, Pekonen F. Ontogenesis of the nuclear 3,5,3’-triiodothyronine receptor in the human fetal brain. Endocrinology. 1984;114(2):677-679. http://dx.doi.org/10.1210/endo-114-2-677.
8. Rastogi RB, Hrdina PD, Dubas T, Singhal RL. Alterations of brain acetylcholine metabolism during neonatal hyperthyroidism. Brain Research. 1977;123(1):188-192. http://dx.doi.org/https://doi.org/10.1016/0006-8993(77)90655-2.
9. Cappola AR, Arnold AM, Wulczyn K, Carlson M, Robbins J, Psaty BM. Thyroid function in the euthyroid range and adverse outcomes in older adults. The Journal of clinical endocrinology and metabolism. 2015;100(3):1088-1096. http://dx.doi.org/10.1210/jc.2014-3586.
10. Pasqualetti G, Pagano G, Rengo G, Ferrara N, Monzani F. Subclinical Hypothyroidism and Cognitive Impairment: Systematic Review and Meta-Analysis. The Journal of clinical endocrinology and metabolism. 2015;100(11):4240-4248. http://dx.doi.org/10.1210/jc.2015-2046.
11. Belakavadi M, Dell J, Grover GJ, Fondell JD. Thyroid hormone suppression of β-amyloid precursor protein gene expression in the brain involves multiple epigenetic regulatory events. Molecular and Cellular Endocrinology. 2011;339(1-2):72-80. http://dx.doi.org/10.1016/j.mce.2011.03.016.
12. Luo L, Yano N, Mao Q, Jackson IM, Stopa EG. Thyrotropin releasing hormone (TRH) in the hippocampus of Alzheimer patients. Journal of Alzheimer’s disease : JAD. 2002;4(2):97-103. http://dx.doi.org/10.3233/jad-2002-4204.
13. Belandia B, Latasa MJ, Villa A, Pascual A. Thyroid hormone negatively regulates the transcriptional activity of the beta-amyloid precursor protein gene. The Journal of biological chemistry. 1998;273(46):30366-30371. http://dx.doi.org/10.1074/jbc.273.46.30366.
14. Serrano-Pozo A, Frosch MP, Masliah E, Hyman BT. Neuropathological alterations in Alzheimer disease. Cold Spring Harb Perspect Med. 2011;1(1):a006189. http://dx.doi.org/10.1101/cshperspect.a006189.
15. Mergenthaler P, Lindauer U, Dienel GA, Meisel A. Sugar for the brain: the role of glucose in physiological and pathological brain function. Trends in neurosciences. 2013;36(10):587-597. http://dx.doi.org/10.1016/j.tins.2013.07.001.
16. Joffe BI, Distiller LA. Diabetes mellitus and hypothyroidism: Strange bedfellows or mutual companions? World J Diabetes. 2014;5(6):901-904. http://dx.doi.org/10.4239/wjd.v5.i6.901.
17. Chaker L, Bianco AC, Jonklaas J, Peeters RP. Hypothyroidism. Lancet (London, England). 2017;390(10101):1550-1562. http://dx.doi.org/10.1016/S0140-6736(17)30703-1.
18. Gaitonde DY, Rowley KD, Sweeney LB. Hypothyroidism: an update. Am Fam Physician. 2012;86(3):244-251.
19. Lahey FH. Non-activated (apathetic) type of hyperthyroidism. New England Journal of Medicine. 1931;204(15):747-748.
20. Thomas FB, Mazzaferri EL, Skillman TG. Apathetic thyrotoxicosis: A distinctive clinical and laboratory entity. Ann Intern Med. 1970;72(5):679-685. http://dx.doi.org/10.7326/0003-4819-72-5-679.
21. Ross DS, Burch HB, Cooper DS, et al. 2016 American Thyroid Association Guidelines for Diagnosis and Management of Hyperthyroidism and Other Causes of Thyrotoxicosis. Thyroid. 2016;26(10):1343-1421. http://dx.doi.org/10.1089/thy.2016.0229.
22. Cooper DS. Clinical practice. Subclinical hypothyroidism. The New England journal of medicine. 2001;345(4):260-265. http://dx.doi.org/10.1056/nejm200107263450406.
23. Biondi B, Cooper DS. Subclinical Hyperthyroidism. The New England journal of medicine. 2018;378(25):2411-2419. http://dx.doi.org/10.1056/NEJMcp1709318.
24. Yeap BB, Alfonso H, Chubb SAP, et al. Higher Free Thyroxine Levels Predict Increased Incidence of Dementia in Older Men: The Health In Men Study. The Journal of Clinical Endocrinology & Metabolism. 2012;97(12):E2230-E2237. http://dx.doi.org/10.1210/jc.2012-2108.
25. Vadiveloo T, Donnan PT, Cochrane L, Leese GP. The Thyroid Epidemiology, Audit, and Research Study (TEARS): morbidity in patients with endogenous subclinical hyperthyroidism. The Journal of clinical endocrinology and metabolism. 2011;96(5):1344-1351. http://dx.doi.org/10.1210/jc.2010-2693.
26. Rieben C, Segna D, da Costa BR, et al. Subclinical Thyroid Dysfunction and the Risk of Cognitive Decline: a Meta-Analysis of Prospective Cohort Studies. The Journal of clinical endocrinology and metabolism. 2016;101(12):4945-4954. http://dx.doi.org/10.1210/jc.2016-2129.
27. Moon JH, Park YJ, Kim TH, et al. Lower-but-normal serum TSH level is associated with the development or progression of cognitive impairment in elderly: Korean Longitudinal Study on Health and Aging (KLoSHA). The Journal of clinical endocrinology and metabolism. 2014;99(2):424-432. http://dx.doi.org/10.1210/jc.2013-3385.
28. Kalmijn S, Mehta KM, Pols HAP, Hofman A, Drexhage HA, Breteler MMB. Subclinical hyperthyroidism and the risk of dementia. The Rotterdam study. Clinical Endocrinology. 2000;53(6):733-737. http://dx.doi.org/10.1046/j.1365-2265.2000.01146.x.
29. Chaker L, Wolters FJ, Bos D, et al. Thyroid function and the risk of dementia: The Rotterdam Study. Neurology. 2016;87(16):1688-1695. http://dx.doi.org/10.1212/wnl.0000000000003227.
30. Annerbo S, Wahlund LO, Lökk J. The significance of thyroid-stimulating hormone and homocysteine in the development of Alzheimer’s disease in mild cognitive impairment: A 6-year follow-up study. American Journal of Alzheimer’s Disease and other Dementias. 2006;21(3):182-188. http://dx.doi.org/10.1177/1533317506289282.
31. Ganguli M, Burmeister LA, Seaberg EC, Belle S, DeKosky ST. Association between dementia and elevated TSH: a community-based study. Biol Psychiatry. 1996;40(8):714-725. http://dx.doi.org/10.1016/0006-3223(95)00489-0.
32. Breteler MM, van Duijn CM, Chandra V, et al. Medical history and the risk of Alzheimer’s disease: a collaborative re-analysis of case-control studies. EURODEM Risk Factors Research Group. International journal of epidemiology. 1991;20 Suppl 2:S36-42. http://dx.doi.org/10.1093/ije/20.supplement_2.s36.
33. Johansson P, Almqvist EG, Johansson JO, et al. Reduced cerebrospinal fluid level of thyroxine in patients with Alzheimer’s disease. Psychoneuroendocrinology. 2013;38(7):1058-1066. http://dx.doi.org/10.1016/j.psyneuen.2012.10.012.
34. Dolatshahi M, Salehipour A, Saghazadeh A, et al. Thyroid hormone levels in Alzheimer disease: a systematic review and meta-analysis. Endocrine. 2022. http://dx.doi.org/10.1007/s12020-022-03190-w.
35. Van Osch LADM, Hogervorst E, Combrinck M, Smith AD. Low thyroid-stimulating hormone as an independent risk factor for Alzheimer disease. Neurology. 2004;62(11):1967-1971. http://dx.doi.org/10.1212/01.WNL.0000128134.84230.9F.
36. de Jong FJ, den Heijer T, Visser TJ, et al. Thyroid hormones, dementia, and atrophy of the medial temporal lobe. The Journal of clinical endocrinology and metabolism. 2006;91(7):2569-2573. http://dx.doi.org/10.1210/jc.2006-0449.
37. Tan ZS, Beiser A, Vasan RS, et al. Thyroid function and the risk of Alzheimer disease: The Framingham study. Archives of Internal Medicine. 2008;168(14):1514-1520. http://dx.doi.org/10.1001/archinte.168.14.1514.
38. Tan ZS, Vasan RS. Thyroid function and alzheimer’s disease. Journal of Alzheimer’s Disease. 2009;16(3):503-507. http://dx.doi.org/10.3233/JAD-2009-0991.
39. Figueroa PBS, Ferreira AFF, Britto LR, Doussoulin AP, Torrão ADS. Association between thyroid function and Alzheimer’s disease: A systematic review. Metab Brain Dis. 2021;36(7):1523-1543. http://dx.doi.org/10.1007/s11011-021-00760-1.
40. Quinlan P, Horvath A, Eckerström C, Wallin A, Svensson J. Altered thyroid hormone profile in patients with Alzheimer’s disease. Psychoneuroendocrinology. 2020;121. http://dx.doi.org/10.1016/j.psyneuen.2020.104844.
41. Yoshimasu F, Kokmen E, Hay ID, Beard CM, Offord KP, Kurland LT. The association between Alzheimer’s disease and thyroid disease in Rochester, Minnesota. Neurology. 1991;41(11):1745-1747. http://dx.doi.org/10.1212/wnl.41.11.1745.
42. Brenowitz WD, Han F, Kukull WA, Nelson PT. Treated hypothyroidism is associated with cerebrovascular disease but not Alzheimer’s disease pathology in older adults. Neurobiology of Aging. 2018;62:64-71. http://dx.doi.org/10.1016/j.neurobiolaging.2017.10.004.
43. Annerbo S, Kivipelto M, Lökk J. A prospective study on the development of alzheimer’s disease with regard to thyroid-stimulating hormone and homocysteine. Dementia and Geriatric Cognitive Disorders. 2009;28(3):275-280. http://dx.doi.org/10.1159/000242439.
44. de Jong FJ, Masaki K, Chen H, et al. Thyroid function, the risk of dementia and neuropathologic changes: the Honolulu-Asia aging study. Neurobiol Aging. 2009;30(4):600-606. http://dx.doi.org/10.1016/j.neurobiolaging.2007.07.019.
45. Stuerenburg HJ, Arlt S, Mueller-Thomsen T. Free thyroxine, cognitive decline and depression in Alzheimer’s disease. Neuroendocrinology Letters. 2006;27(4):535-537.
46. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. http://dx.doi.org/10.1371/journal.pmed.1000097.
47. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603-605. http://dx.doi.org/10.1007/s10654-010-9491-z.
48. Higgins JP. Cochrane handbook for systematic reviews of interventions version 5.0. 1. The Cochrane Collaboration. http://www cochrane-handbook org. 2008.
49. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Bmj. 2003;327(7414):557-560.
50. Small GW, Matsuyama SS, Komanduri R, Kumar V, Jarvik LF. Thyroid disease in patients with dementia of the Alzheimer type. Journal of the American Geriatrics Society. 1985;33(8):538-539.
51. Lopez O, Huff FJ, Martinez AJ, Bedetti CD. Prevalence of thyroid abnormalities is not increased in Alzheimer’s disease. Neurobiology of Aging. 1989;10(3):247-251. http://dx.doi.org/10.1016/0197-4580(89)90058-4.
52. Cárdenas-Ibarra L, Solano-Velázquez JA, Salinas-Martínez R, Aspera-Ledezma TD, Sifuentes-Martínez MdR, Villarreal-Pérez JZ. Cross-sectional observations of thyroid function in geriatric Mexican outpatients with and without dementia. Archives of Gerontology and Geriatrics. 2008;46(2):173-180. http://dx.doi.org/10.1016/j.archger.2007.03.009.
53. Ranzola RM, Rodríguez YR, Cuesta JJ, Truffiz AP, Llano JC. Myeloperoxidase activity, lipid profile and thyroid function in patients who suffer from alzheimer´s disease. Revista Cubana de Investigaciones Biomedicas. 2019;38(1).
54. Heun R, Schoepf D, Potluri R, Natalwala A. Alzheimer’s disease and co-morbidity: increased prevalence and possible risk factors of excess mortality in a naturalistic 7-year follow-up. European psychiatry : the journal of the Association of European Psychiatrists. 2013;28(1):40-48. http://dx.doi.org/10.1016/j.eurpsy.2011.06.001.
55. Hu Y, Wang ZC, Guo QH, Cheng W, Chen YW. Is thyroid status associated with cognitive impairment in elderly patients in China? BMC Endocrine Disorders. 2016;16(1). http://dx.doi.org/10.1186/s12902-016-0092-z.
56. Suhanov AV, Pilipenko PI, Korczyn AD, et al. Risk factors for Alzheimer’s disease in Russia: a case-control study. Eur J Neurol. 2006;13(9):990-995. http://dx.doi.org/10.1111/j.1468-1331.2006.01391.x.
57. Prezioso G, Giannini C, Chiarelli F. Effect of Thyroid Hormones on Neurons and Neurodevelopment. Hormone research in paediatrics. 2018;90(2):73-81. http://dx.doi.org/10.1159/000492129.
58. Ambrogini P, Cuppini R, Ferri P, et al. Thyroid hormones affect neurogenesis in the dentate gyrus of adult rat. Neuroendocrinology. 2005;81(4):244-253. http://dx.doi.org/10.1159/000087648.
59. Madeira MD, Sousa N, Lima-Andrade MT, Calheiros F, Cadete-Leite A, Paula-Barbosa MM. Selective vulnerability of the hippocampal pyramidal neurons to hypothyroidism in male and female rats. The Journal of comparative neurology. 1992;322(4):501-518. http://dx.doi.org/10.1002/cne.903220405.
60. Alzoubi KH, Gerges NZ, Aleisa AM, Alkadhi KA. Levothyroxin restores hypothyroidism-induced impairment of hippocampus-dependent learning and memory: Behavioral, electrophysiological, and molecular studies. Hippocampus. 2009;19(1):66-78. http://dx.doi.org/10.1002/hipo.20476.
61. Torres-Manzo AP, Franco-Colín M, Blas-Valdivia V, Pineda-Reynoso M, Cano-Europa E. Hypothyroidism Causes Endoplasmic Reticulum Stress in Adult Rat Hippocampus: A Mechanism Associated with Hippocampal Damage. Oxidative medicine and cellular longevity. 2018;2018:2089404-2089404. http://dx.doi.org/10.1155/2018/2089404.
62. Marouli E, Yusuf L, Kjaergaard AD, et al. Thyroid Function and the Risk of Alzheimer’s Disease: A Mendelian Randomization Study. Thyroid. 2021;31(12):1794-1799. http://dx.doi.org/10.1089/thy.2021.0321.
63. Li GH, Cheung CL, Cheung EY, Chan WC, Tan KC. Genetically Determined TSH Level Within Reference Range Is Inversely Associated With Alzheimer Disease. The Journal of clinical endocrinology and metabolism. 2021;106(12):e5064-e5074. http://dx.doi.org/10.1210/clinem/dgab527.
64. Latasa MJ, Belandia B, Pascual A. Thyroid hormones regulate β-amyloid gene splicing and protein secretion in neuroblastoma cells. Endocrinology. 1998;139(6):2692-2698. http://dx.doi.org/10.1210/endo.139.6.6033.
65. Panveloski-Costa AC, Silva Teixeira S, Ribeiro IM, et al. Thyroid hormone reduces inflammatory cytokines improving glycaemia control in alloxan-induced diabetic wistar rats. Acta physiologica (Oxford, England). 2016;217(2):130-140. http://dx.doi.org/10.1111/apha.12647.
66. Santos MCS, Louzada RAN, Souza ECL, et al. Diabetes Mellitus Increases Reactive Oxygen Species Production in the Thyroid of Male Rats. Endocrinology. 2013;154(3):1361-1372. http://dx.doi.org/10.1210/en.2012-1930.
67. Mosconi L. Glucose metabolism in normal aging and Alzheimer’s disease: Methodological and physiological considerations for PET studies. Clin Transl Imaging. 2013;1(4). http://dx.doi.org/10.1007/s40336-013-0026-y.
68. Heni M, Kullmann S, Preissl H, Fritsche A, Häring HU. Impaired insulin action in the human brain: causes and metabolic consequences. Nature reviews Endocrinology. 2015;11(12):701-711. http://dx.doi.org/10.1038/nrendo.2015.173.
69. Moosavi M, Naghdi N, Maghsoudi N, Zahedi Asl S. The effect of intrahippocampal insulin microinjection on spatial learning and memory. Horm Behav. 2006;50(5):748-752. http://dx.doi.org/10.1016/j.yhbeh.2006.06.025.
70. de la Monte SM, Wands JR. Review of insulin and insulin-like growth factor expression, signaling, and malfunction in the central nervous system: relevance to Alzheimer’s disease. Journal of Alzheimer’s disease : JAD. 2005;7(1):45-61. http://dx.doi.org/10.3233/jad-2005-7106.
71. Hölscher C. Insulin Signaling Impairment in the Brain as a Risk Factor in Alzheimer’s Disease. Front Aging Neurosci. 2019;11:88. http://dx.doi.org/10.3389/fnagi.2019.00088.
72. Chen JM, Huang CQ, Ai M, Kuang L. Circadian rhythm of TSH levels in subjects with Alzheimer’s disease (AD). Aging Clinical and Experimental Research. 2013;25(2):153-157. http://dx.doi.org/10.1007/s40520-013-0025-x.
73. Hebda-Bauer EK, Simmons TA, Sugg A, et al. 3xTg-AD mice exhibit an activated central stress axis during early-stage pathology. Journal of Alzheimer’s disease : JAD. 2013;33(2):407-422. http://dx.doi.org/10.3233/jad-2012-121438.
74. Ahmad MH, Fatima M, Mondal AC. Role of Hypothalamic-Pituitary-Adrenal Axis, Hypothalamic-Pituitary-Gonadal Axis and Insulin Signaling in the Pathophysiology of Alzheimer’s Disease. Neuropsychobiology. 2019;77(4):197-205. http://dx.doi.org/10.1159/000495521.
75. Yong-Hong L, Xiao-Dong P, Chang-Quan H, Bo Y, Qing-Xiu L. Hypothalamic-pituitary-thyroid axis in patients with Alzheimer disease (AD). Journal of investigative medicine : the official publication of the American Federation for Clinical Research. 2013;61(3):578-581. http://dx.doi.org/10.2310/JIM.0b013e318280aafb.
76. Popp J, Wolfsgruber S, Heuser I, et al. Cerebrospinal fluid cortisol and clinical disease progression in MCI and dementia of Alzheimer’s type. Neurobiol Aging. 2015;36(2):601-607. http://dx.doi.org/10.1016/j.neurobiolaging.2014.10.031.
77. Du X, Pang TY. Is Dysregulation of the HPA-Axis a Core Pathophysiology Mediating Co-Morbid Depression in Neurodegenerative Diseases? Frontiers in psychiatry. 2015;6:32. http://dx.doi.org/10.3389/fpsyt.2015.00032.
78. Montero-Pedrazuela A, Venero C, Lavado-Autric R, et al. Modulation of adult hippocampal neurogenesis by thyroid hormones: implications in depressive-like behavior. Mol Psychiatry. 2006;11(4):361-371. http://dx.doi.org/10.1038/sj.mp.4001802.
79. Thompson CC, Potter GB. Thyroid Hormone Action in Neural Development. Cerebral Cortex. 2000;10(10):939-945. http://dx.doi.org/10.1093/cercor/10.10.939.
80. Chaalal A, Poirier R, Blum D, Laroche S, Enderlin V. Thyroid Hormone Supplementation Restores Spatial Memory, Hippocampal Markers of Neuroinflammation, Plasticity-Related Signaling Molecules, and β-Amyloid Peptide Load in Hypothyroid Rats. Mol Neurobiol. 2019;56(1):722-735. http://dx.doi.org/10.1007/s12035-018-1111-z.
81. Li LX, Yang T, Guo L, et al. Serum tau levels are increased in patients with hyperthyroidism. Neuroscience Letters. 2020;729. http://dx.doi.org/10.1016/j.neulet.2020.135003.
82. Goumidi L, Flamant F, Lendon C, et al. Study of thyroid hormone receptor alpha gene polymorphisms on Alzheimer’s disease. Neurobiology of Aging. 2011;32(4):624-630. http://dx.doi.org/10.1016/j.neurobiolaging.2009.04.007.
83. Delivoria DC, Skretas G. The Discovery of Peptide Macrocycle Rescuers of Pathogenic Protein Misfolding and Aggregation by Integrating SICLOPPS Technology and Ultrahigh-Throughput Screening in Bacteria. Methods in molecular biology (Clifton, NJ). 2022;2371:215-246. http://dx.doi.org/10.1007/978-1-0716-1689-5_12.
84. Rich MW. Hypothyroidism in association with systemic amyloidosis. Head & neck. 1995;17(4):343-345. http://dx.doi.org/10.1002/hed.2880170412.
85. Prieto-Almeida F, Panveloski-Costa AC, Crunfli F, da Silva Teixeira S, Nunes MT, Torrão ADS. Thyroid hormone improves insulin signaling and reduces the activation of neurodegenerative pathway in the hippocampus of diabetic adult male rats. Life Sciences. 2018;192:253-258. http://dx.doi.org/10.1016/j.lfs.2017.11.013
86. Chaalal A, Poirier R, Blum D, et al. PTU-induced hypothyroidism in rats leads to several early neuropathological signs of Alzheimer’s disease in the hippocampus and spatial memory impairments. Hippocampus. 2014;24(11):1381-1393. http://dx.doi.org/10.1002/hipo.22319.
87. McDermott MT, Ridgway EC. Subclinical hypothyroidism is mild thyroid failure and should be treated. The Journal of clinical endocrinology and metabolism. 2001;86(10):4585-4590. http://dx.doi.org/10.1210/jcem.86.10.7959.
88. Laurberg P, Andersen S, Bülow Pedersen I, Carlé A. Hypothyroidism in the elderly: pathophysiology, diagnosis and treatment. Drugs Aging. 2005;22(1):23-38. http://dx.doi.org/10.2165/00002512-200522010-00002.
89. Stott DJ, Rodondi N, Kearney PM, et al. Thyroid Hormone Therapy for Older Adults with Subclinical Hypothyroidism. The New England journal of medicine. 2017;376(26):2534-2544. http://dx.doi.org/10.1056/NEJMoa1603825.
90. Benseñor IM, Lotufo PA, Menezes PR, Scazufca M. Subclinical hyperthyroidism and dementia: the Sao Paulo Ageing & Health Study (SPAH). BMC Public Health. 2010;10:298. http://dx.doi.org/10.1186/1471-2458-10-298.
91. Videla LA, Sir T, Wolff C. Increased lipid peroxidation in hyperthyroid patients: suppression by propylthiouracil treatment. Free radical research communications. 1988;5(1):1-10. http://dx.doi.org/10.3109/10715768809068553.
92. Chan RS, Huey ED, Maecker HL, et al. Endocrine modulators of necrotic neuron death. Brain pathology (Zurich, Switzerland). 1996;6(4):481-491. http://dx.doi.org/10.1111/j.1750-3639.1996.tb00877.x.
93. Cummings BJ, Cotman CW. Image analysis of beta-amyloid load in Alzheimer’s disease and relation to dementia severity. Lancet. 1995;346(8989):1524-1528. http://dx.doi.org/10.1016/s0140-6736(95)92053-6.
94. Schmitt TL, Steiner E, Klingler P, Lassmann H, Grubeck-Loebenstein B. Thyroid epithelial cells produce large amounts of the Alzheimer beta-amyloid precursor protein (APP) and generate potentially amyloidogenic APP fragments. The Journal of clinical endocrinology and metabolism. 1995;80(12):3513-3519. http://dx.doi.org/10.1210/jcem.80.12.8530592.
95. Ehrenkranz J, Bach PR, Snow GL, et al. Circadian and Circannual Rhythms in Thyroid Hormones: Determining the TSH and Free T4 Reference Intervals Based Upon Time of Day, Age, and Sex. Thyroid. 2015;25(8):954-961. http://dx.doi.org/10.1089/thy.2014.0589.
96. Boucai L, Hollowell JG, Surks MI. An approach for development of age-, gender-, and ethnicity-specific thyrotropin reference limits. Thyroid. 2011;21(1):5-11. http://dx.doi.org/10.1089/thy.2010.0092.
97. Raverot V, Bonjour M, Abeillon du Payrat J, et al. Age- and Sex-Specific TSH Upper-Limit Reference Intervals in the General French Population: There Is a Need to Adjust Our Actual Practices. J Clin Med. 2020;9(3). http://dx.doi.org/10.3390/jcm9030792.
98. Surks MI, Hollowell JG. Age-specific distribution of serum thyrotropin and antithyroid antibodies in the US population: implications for the prevalence of subclinical hypothyroidism. The Journal of clinical endocrinology and metabolism. 2007;92(12):4575-4582. http://dx.doi.org/10.1210/jc.2007-1499.
99. Vadiveloo T, Donnan PT, Murphy MJ, Leese GP. Age- and gender-specific TSH reference intervals in people with no obvious thyroid disease in Tayside, Scotland: the Thyroid Epidemiology, Audit, and Research Study (TEARS). The Journal of clinical endocrinology and metabolism. 2013;98(3):1147-1153. http://dx.doi.org/10.1210/jc.2012-3191.
100. Yoshihara A, Noh JY, Watanabe N, et al. Seasonal Changes in Serum Thyrotropin Concentrations Observed from Big Data Obtained During Six Consecutive Years from 2010 to 2015 at a Single Hospital in Japan. Thyroid. 2018;28(4):429-436. http://dx.doi.org/10.1089/thy.2017.0600.
© The Authors 2023