S. Mattke1, H. Jun2, M. Hanson1, S. Chu3, J.H. Kordower4, E.M. Reiman5
1. The USC Brain Health Observatory, University of Southern California, Los Angeles, CA 90089, USA; 2. Harvard Medical School, Boston, MA 02115, USA; 3. Cornell University, Ithaca, NY 14850; 4. Arizona State University-Banner Neurodegenerative Disease Research Center, Tempe, AZ 85281, USA; 5. Banner Alzheimer’s Institute, Phoenix, AZ 85006
Corresponding Author: Soeren Mattke, M.D., D.Sc., Director, Center for Improving Chronic Illness Care, Research Professor of Economics, USC Dornsife, 635 Downey Way, #505N, Los Angeles, CA 90089, Mobile: +1 202 468 5797, mattke@usc.edu
J Prev Alz Dis 2024;2(11):303-309
Published online January 30, 2024, http://dx.doi.org/10.14283/jpad.2024.23
Abstract
INTRODUCTION: As treatments for secondary prevention of Alzheimer’s disease (AD) are being studied, concerns about their value for money have appeared. We estimate cost-effectiveness of a hypothetical screening and prevention program.
METHODS: We use a Markov model to project cost-effectiveness of a treatment that reduces progression to symptomatic AD by 50% with either chronic treatment until progression to mild cognitive impairment or treatment for one year followed by monitoring with AD blood tests and retreatment with one dose in case of amyloid re-accumulation. Diagnoses would be made with an AD blood test with sensitivity and specificity of 80%, and inconclusive results in 20%. Individuals testing negative would be re-tested in five years and those with inconclusive results in one.
RESULTS: The program would generate per-person value of $53,721 from a payer (reduction of direct cost and patient QALY gains) and $69,861 from a societal perspective (adding valuation of reduced caregiver burden). With chronic treatment, it would be cost-effective up to annual drug prices of $7,000 and $10,300, respectively. Time-limited treatment would be cost-effective at annual drug prices of $54,257 and $78,458 from a payer and societal perspective, respectively. Higher specificity of the blood test would decrease cost per person with similar value generation
DISCUSSION: A hypothetical prevention treatment for AD could be economically viable from a payer and societal perspective.
Key words: Alzheimer’s disease, prevention, amyloid, cost-effectiveness, modeling, value.
Introduction
The National Plan to Address Alzheimer’s Disease (AD) has sought to find an effective prevention therapy by 2025. In addition to efficacy, a prevention therapy, including the necessary screening program, must to generate adequate value for money, as payers would be unlikely to cover such a program, if cost were too high relative to benefit.
Phase 3 trials for lecanemab (1) and donanemab (2), both monoclonal antibodies targeting amyloid-β (Aβ) plaques, have shown a reduction of clinical and functional decline in patients with mild cognitive impairment (MCI) or mild dementia due to Alzheimer’s disease (AD). These results support the hypothesis that Aβ aggregates are involved in the development, treatment and potential prevention of AD; and increase the chance that ongoing secondary prevention trials of lecanemab (3) (AHEAD 3-45) and donanemab (TRAILBLAZER-ALZ 3) will show a significant benefit. These trials investigate these drugs in cognitively unimpaired persons with PET and/or plasma biomarker evidence of Aβ plaques, and, if positive, would support the accelerated approval of an AD prevention therapy within the next few years.
Since evidence of Aβ plaques begins about 10-20 years before the onset of MCI, by which time disease burden is already extensive, there is reason to believe that the initiation of a secondary AD prevention therapy in cognitively unimpaired persons with Aβ plaques could have a more profound impact in slowing disease progression and preventing the onset of symptoms. Moreover, since the costs and burden to patients and families are already significant after the onset of cognitive impairment (4) , imposing a natural limit on the benefit, an Aβ-directed therapy that is initiated before the onset of impairment could have a greater economic benefit. An analysis of the GERAS-US data by Robinson et al. (5), for example, reported monthly medical and social care cost associated with MCI of $1,098 and $194 in 2017 USD, respectively.
While these estimates suggest substantial potential gross value for a preventive treatment, the net benefit, and thus the economic viability of a prevention program, will be a function of cost for diagnosis, drug, and monitoring compared to cost offsets and gains in quality-adjusted life-years (QALYs), i.e., the life-years gained from the treatment adjusted for severity of illness in those years. On the one hand, the complexity and likely cost of Aβ-targeting treatments, which entail biomarker testing, potential infusion delivery, monitoring for ARIA and patented drugs, are clearly quite different from many other preventive treatments, like flu shots and cholesterol-lowering drugs. On the other, fairly complex screening and prevention programs, such as serial CT scans in smokers for lung cancer (6) and colonoscopy screening for colorectal malignancies (7), have been shown to generate adequate value for money and are recommended by the US Preventive Services Task Force.
Understanding and debating the contours of a secondary prevention program, which would necessitate screening for the AD pathology given the limited or absent subjective symptoms at that disease state, well ahead of an approval of a treatment has substantial policy implications because of the large size of the potentially eligible population. According meta-analyses of PET and CSF data from nearly 10,000 persons (8), about a quarter of cognitively unimpaired persons with an average age of about 70 have biomarker evidence of Aβ plaques.
In this article, we conduct a thought experiment and assess a hypothetical blood-based biomarker screening program to identify cognitively unimpaired persons with biomarker evidence of Aβ plaques and estimate per person costs and benefits of a population-based AD prevention therapy, with Aβ plaque-lowering antibodies. We explore a range of assumptions with a decision-analytic model to determine the economic viability of such a program. Of note, our expectation is not that the program would reduce net spending on medical and social care cost, as this would be highly unusual for medical interventions, but to create adequate value for money, i.e., to be cost-effective rather than cost saving. We consider the payer perspective that takes direct cost offsets and an implied valuation of gains in quality adjusted life-years (QALYs) into account and the societal perspective that also considers reduced caregiver burden.
Methods
Hypothetical care pathway
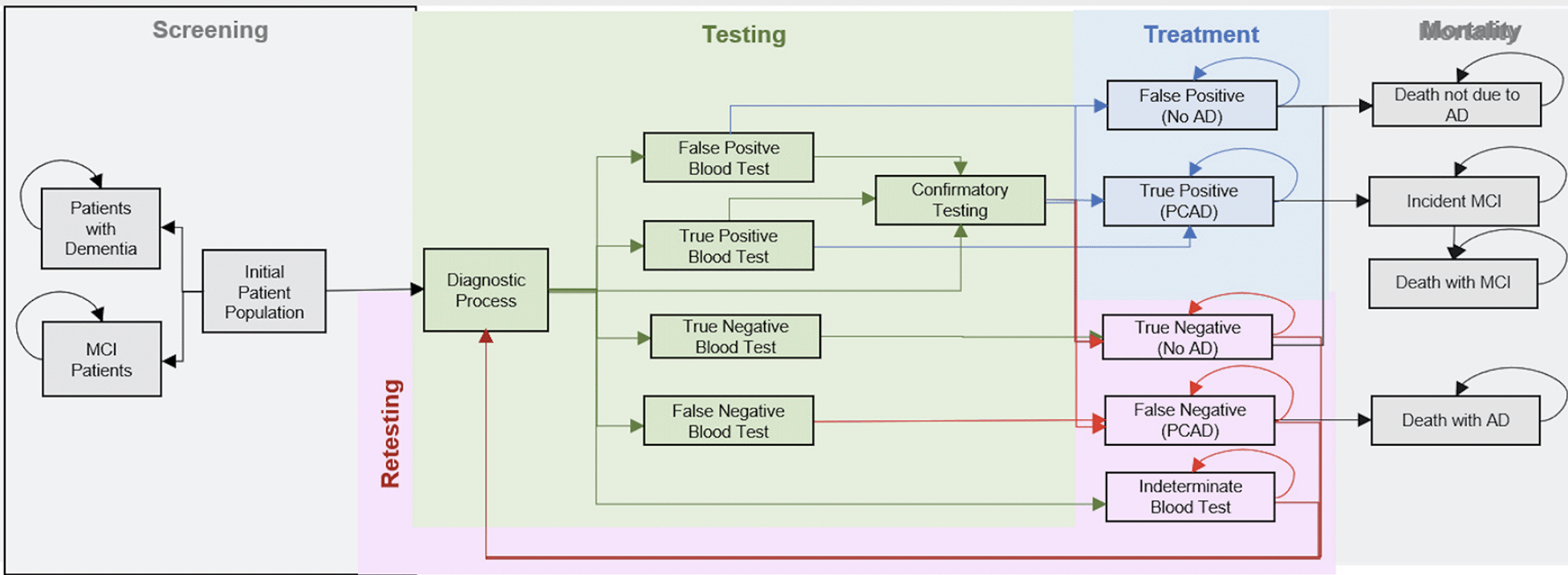
Here, we considered the 2022 U.S. cohort of adults aged between 55 and 79 without cognitive impairment, and we project their outcomes over a 30-year horizon. As illustrated in Figure 1, every individual in this cohort would receive a blood test for the Alzheimer’s pathology in a primary care setting. The blood test can be positive for the Alzheimer’s pathology, negative or indeterminate, and we assume a sensitivity and specificity of 80% for conclusive results, whereas indeterminate tests do not carry information, i.e., sensitivity and specificity are only calculated for the share of conclusive test results. We assume the 20% of individuals with indeterminate test results would not undergo further assessment and return for retesting in the subsequent years until the test is conclusively either positive or negative. We assume that four out of five Individuals with a conclusively positive test result would be directly referred to treatment, whereas the remaining 20% would undergo confirmatory testing with PET scan (50%) or CSF analysis (50%) with sensitivity and specificity of 95% and be referred to treatment based on those results. Individuals testing negative at any stage of the diagnostic process would return for another blood test in five years. No further testing would be undertaken once an individual turns 80 years of age. The upper age limit was imposed in keeping with other screening programs, such as for colorectal and lung cancer, as advanced age would make it less likely to see a net clinical benefit.
Our base case assumption is that the treatment would reduce the progression from preclinical AD to MCI due to AD by 50% as an estimate of effectiveness, i.e., accounting for treatment interruptions and discontinuations under real-world conditions. The treatment would have no direct effect on mortality, because preclinical AD is not associated with higher mortality. Indirect effects on mortality would come from a delayed or avoided onset of MCI, i.e., the impact on life-years and QALYs is based on delaying the progression to symptomatic disease stages with elevated mortality risk.
Model structure, parameters and sources
Disease burden and transition probabilities
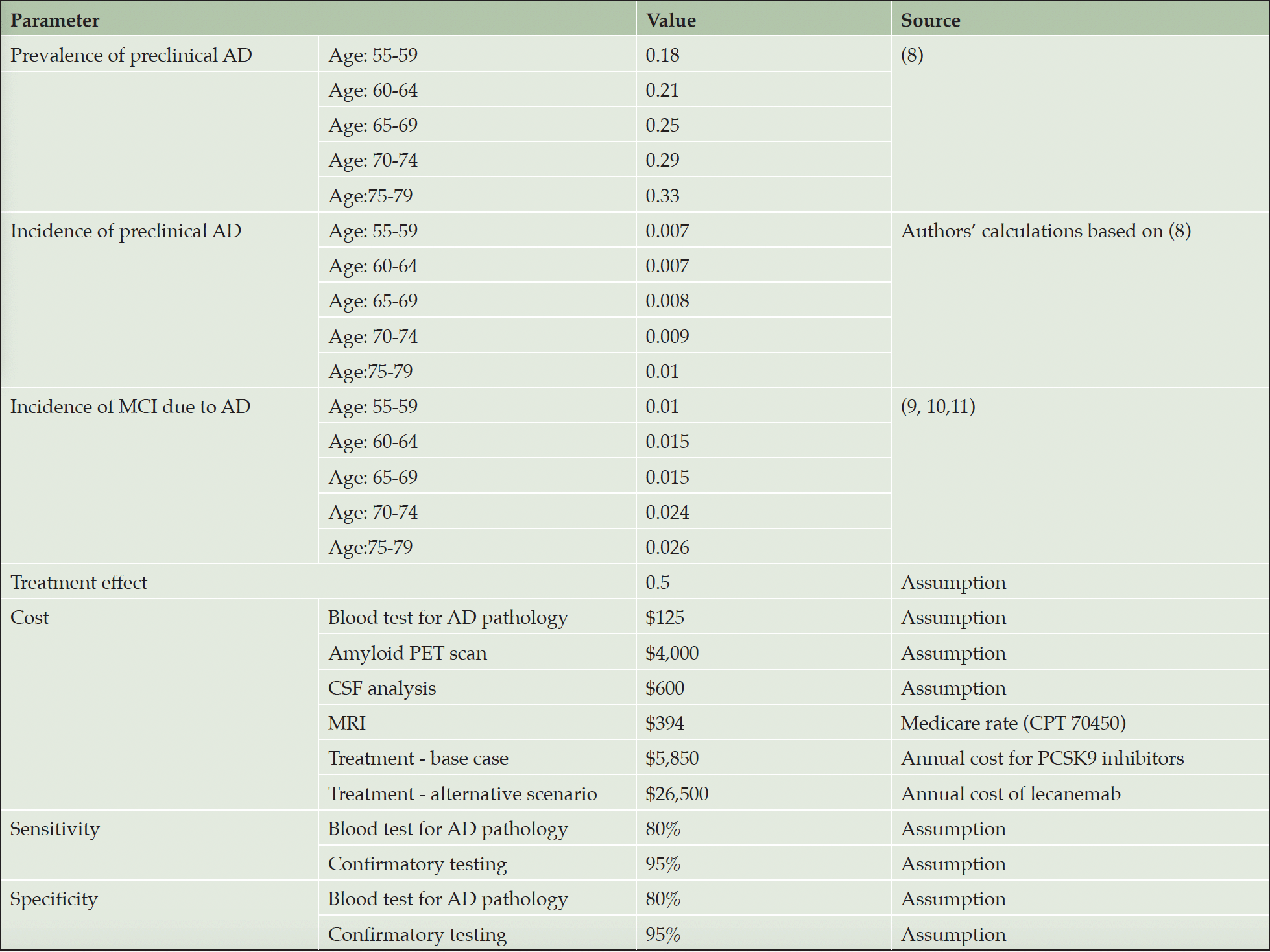
Estimates for the prevalence of preclinical Alzheimer’s disease (i.e., biomarker evidence of Aβ plaques in cognitively unimpaired persons in this age group) were based on Jansen et al., which also served as source for the derivation of incidence (8). We used population counts and corresponding mortality rates in five-year age brackets from 2020 U.S. Census and calculated (1) the number of amyloid positive cases based on the Jansen data, and (2) the number of individuals that survived 5 years. This allowed us to generate the number of amyloid positive cases alive at the last year of the age bracket after accounting for 5-year mortality. Subtracting those cases from the prevalent cases in the next age bracket gave us the number of incident cases in that bracket. From this, we calculated the annual incidence (reported in cases by person-years) as a population-weighted average. Transition probabilities from preclinical AD to MCI were obtained from a 2019 meta-analysis by Parnetti et al. (9) and recent studies by Cho et al. (10) and Roberts et al. (11) (Table 1).
We used a recent publication Prados et al. (4) to derive estimates for the cost and indirect mortality effects of delaying the onset of MCI. They projected the long-term societal value of a treatment that delayed AD progression from the MCI stage by 30% and estimated as counterfactual the impact of the natural history of MCI due to AD in the absence of treatment, which provided us with estimates for the impact of MCI onset to measure the value of delaying it. The parameters for this model are documented in the Appendix, Tables A1 and A2.
Costs
We assume that the AD blood test would cost $125, including the venipuncture, a PET scan $4,000, and a CSF test $600, including the lumbar puncture. We used two assumptions for the cost of the treatment. The first, our base case, was $5,850 per year using the 2023 list prices of the PCSK9 inhibitors as a proxy, since those are also monoclonal antibodies used in secondary prevention. The treatment would be used until transition to MCI.
The second scenario assumed annual cost of $26,500 based on the list price of lecanemab. This treatment would be used for 12 months, similar to donanemab (2), with one PET scan to confirm clearance, followed by annual blood tests to detect re-accumulation of amyloid deposits. Based on recently published data (12), we assume average time to re-accumulation to be five years and retreatment with one dose.
We assumed four MRIs without contrast at baseline and at months 6, 12 and 18 for ARIA monitoring, following the aducanumab label (13) and recent appropriate use recommendations (14) at the Medicare rate of $394 per scan. All cost estimates are expressed in 2023 US$ and inflated by three percent annually in subsequent years.
Benefits
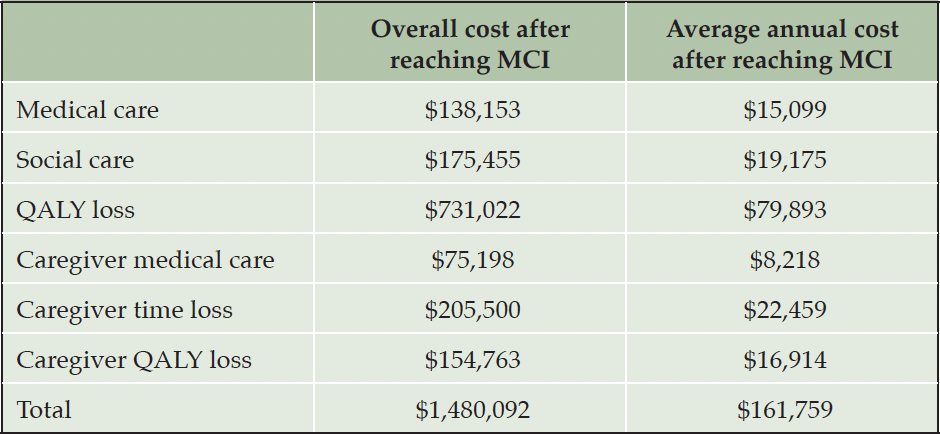
Estimates for medical and social care cost, caregiver burden, which includes time loss, QALY loss and added medical cost, and patients’ QALY loss after progression to MCI were obtained from Prados et al. (4), who estimated the impact of the natural history of MCI in the absence of treatment, as described above. Loss of QALYs for individuals with MCI was based on their estimates of time spent in stages of cognitive impairment of increasing severity plus estimates of average loss of five life-years due to MCI (15, 16). QALYs were valued at $150,000 and future savings and QALY gains discounted by 3% per year (Table 2).
Note: Estimates were derived from the projection of the impact of the natural history of MCI due to AD by Prados et al. (4) and inflated to US$20231
Results
Base case
Under our assumptions for test performance and treatment effectiveness, 31,978,527 Americans out of an eligible starting cohort of 74,951,803 would be treated over the 30-year model horizon with an average treatment duration of 12 years and 5,746,768 incident MCI cases avoided, as individuals would die before progression to MCI.
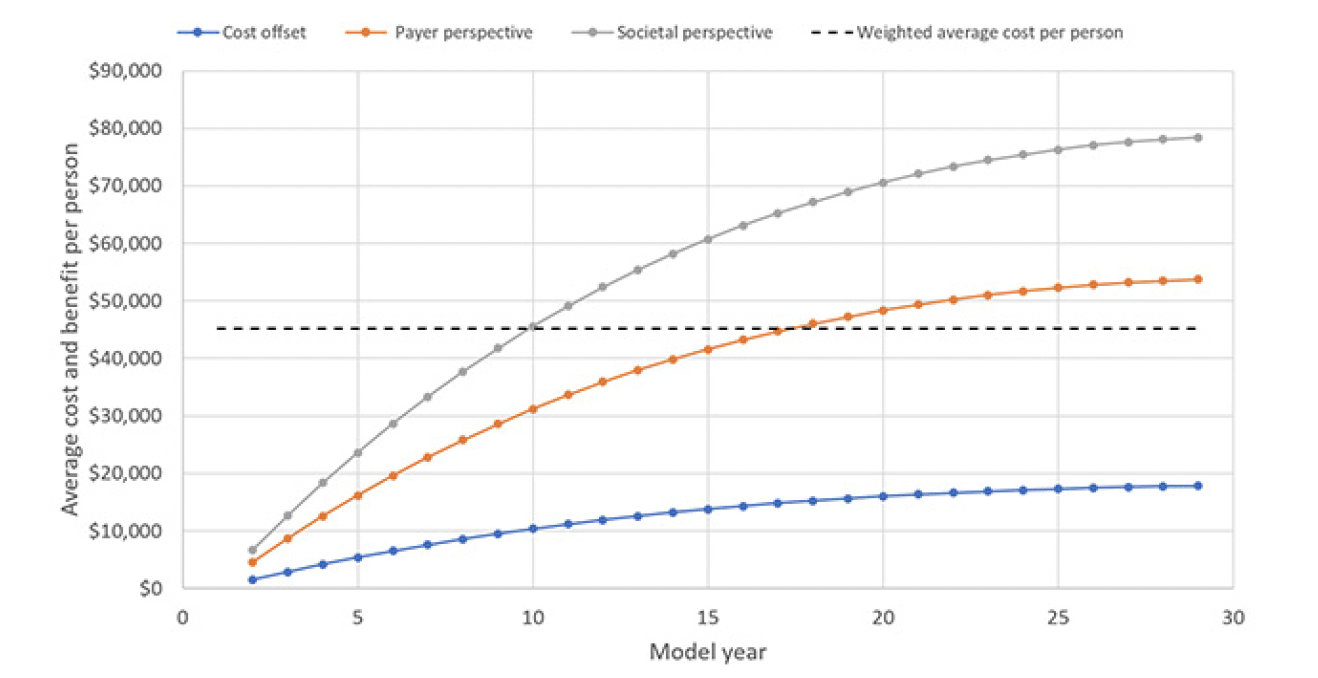
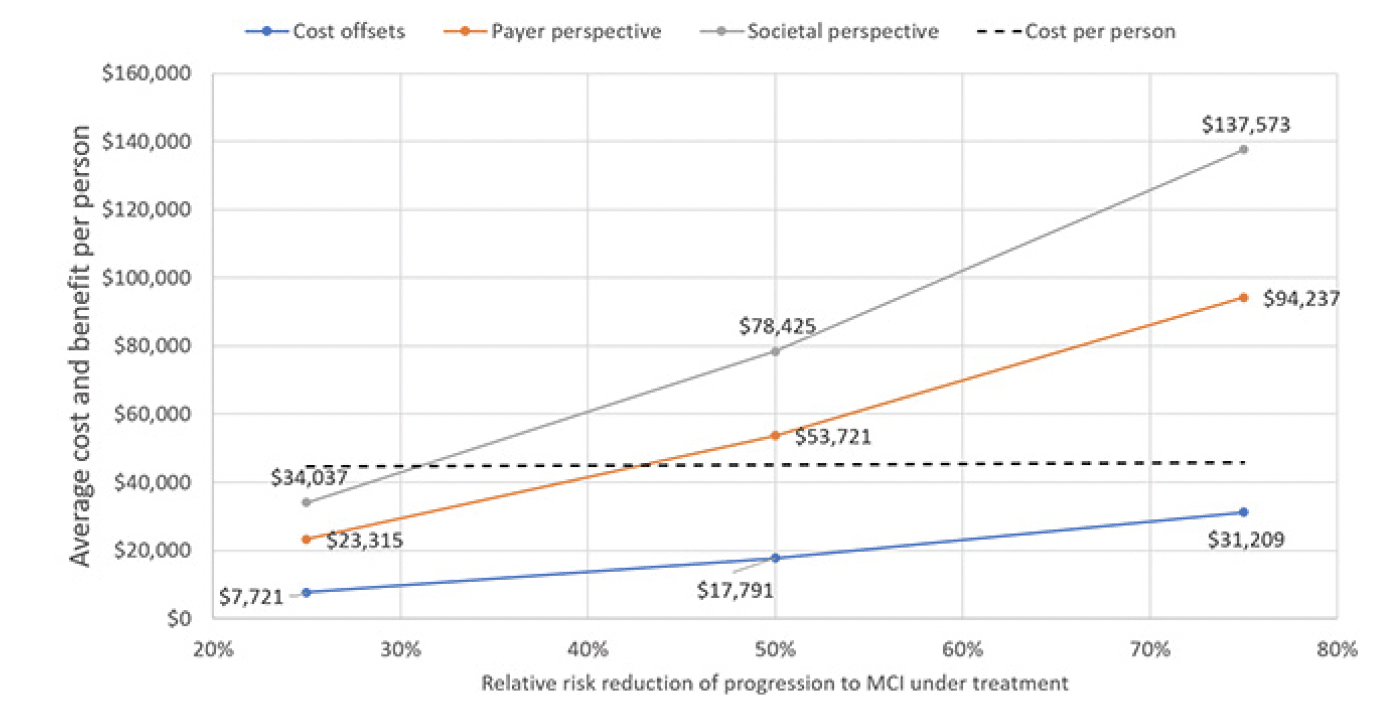
Using the list price of the PCSK9 inhibitors of $5,850 per year, the average cost per included person would be $45,157, with 98.6% going toward cost of the treatment and only 0.6% each toward blood testing and confirmatory testing for the Alzheimer’s pathology. Figure 2 displays the trajectory of costs and benefits under this scenario. Costs offsets reflect the avoided cost for professionally provided medical and social care, the payer perspective adds the valuation of avoided MCI cases’ QALY gains at $150,000 per person, which is the typical assumption for payers in the U.S., and the societal perspective also adds the value of reduced caregiver burden, defined as attributable medical cost and the valuation of caregivers’ lost QALYs, earnings and leisure time. The results suggest that the prevention program would be cost-effective from a payer perspective over the model horizon with total value of $53,721 of which $17,791 stems from avoided cost of care. The program is projected to reach cost-effectiveness in Year 18, when the value trajectory from a payer perspective crosses the average cost trajectory in Figure 2. From a societal perspective, the program would generate $24,704 in net value, i.e., value less treatment cost, per person and reach cost-effectiveness in Year 10.
Effect of assumptions for treatment cost
Figure 3 illustrates how the cost effectiveness of the treatment would fare under different assumptions for treatment cost. At our current assumptions, the program would no longer be cost-effective from a payer and a societal perspective, if the average cost per person were over around $54,000 and $78,000, respectively, which corresponds to a drug price of around $7,000 and $10,300 for a year of treatment. Thus, chronic treatment at an annual cost of $26,500 would not be cost-effective from either perspective.
Our second assumption, time-limited treatment for one year and subsequent retreatment in case of amyloid re-accumulation, is shown in Figure 4. In this scenario, the program would be cost-effective at an annual cost of the treatment of $26,500, which corresponds to an overall average price per year of $25,303. The annual cost of the treatment would have to drop to around ~$18,000 for the program to become cost-neutral with $17,956 cost per person. Up to an annual treatment cost of $60,000 (overall cost of $54.257) and $88,000 (overall cost of $78,458) the program would remain cost-effective from a payer and societal perspective, respectively.
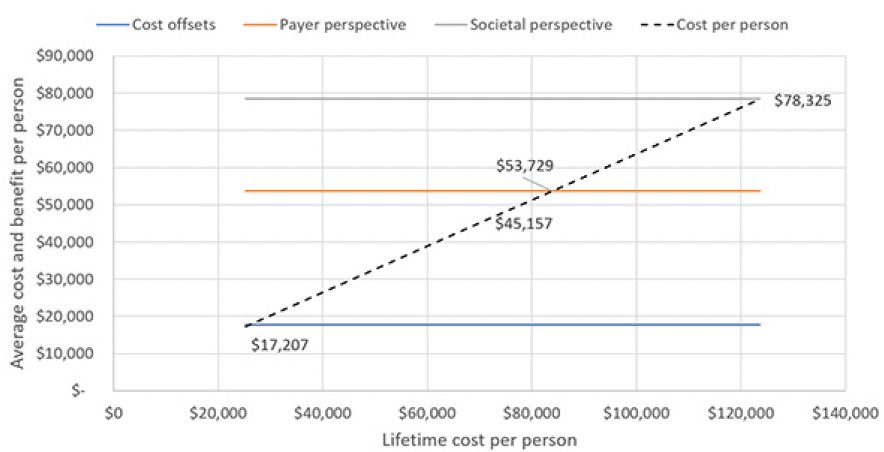
Figure 3. Breakeven analysis for different assumptions for size of treatment effect – base case assumption for treatment cost
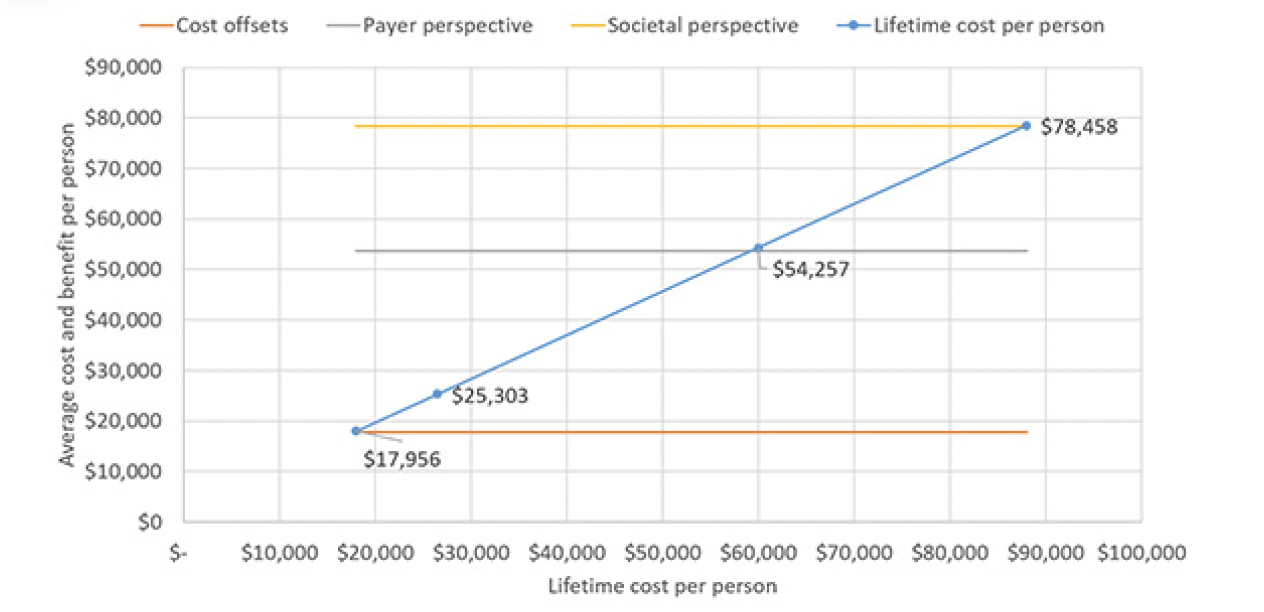
Figure 4. Breakeven analysis for different assumptions for size of treatment effect – alternative assumption for treatment cost
Effect of treatment effectiveness
Figure 5 illustrates how different assumptions for the treatment effect would influence the projection of overall value, again using the first assumption for annual treatment cost. If the treatment delayed progression to MCI by only 25 percent, the program would no longer be cost-effective from a payer or societal perspective as the value would decline to $23,315 and $34,037, respectively. Not even an effect size of 80 percent would make the program cost-neutral as it would generate $31,209 in direct cost offsets and the treatment would have to eliminate the progression from preclinical AD to MCI to become cost-neutral.
Effect of blood test performance
Improving the specificity of the blood test decreases the number of false positives referred to treatment and hence the per person cost as Figure 6 illustrates. Cost per person decreases linearly from $53,354 at a specificity of 70 percent to $31,614 at a specificity of 95 percent, whereas generated value remains nearly unchanged (left panel). The right panel of Figure 6 shows that average cost per person increases with the sensitivity of the blood test, whereas generated value decreases.
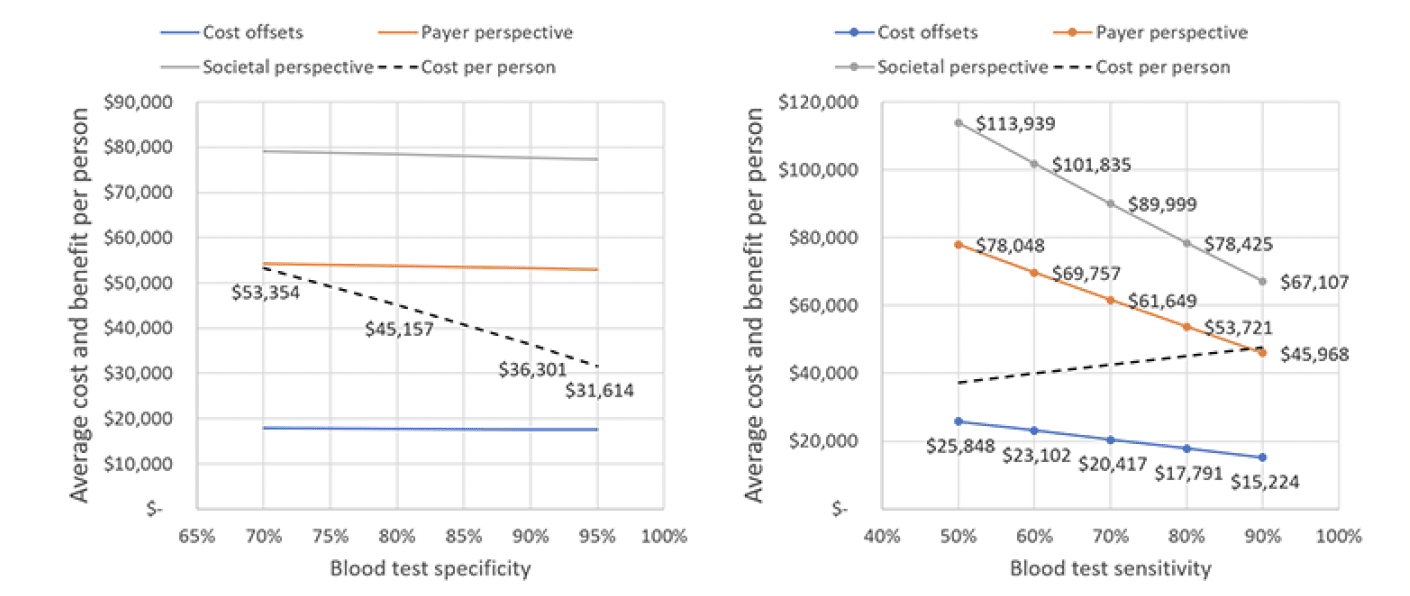
Figure 6. Effect of blood test specificity (left panel) and specificity (right panel) on cost and value per person
Discussion
Our results suggest that a preventive treatment that reduces the rate of progression from preclinical AD to MCI by 50% would be economically viable from a payer perspective over a range of assumptions, albeit not cost-saving, as is typically the case for prevention programs. The economic value, which such a prevention program could generate, is considerably higher than that of a treatment for early-stage symptomatic disease with a comparable effect on progression. Reduced medical and social care cost were projected to be $24,797 and $31,560 per treated person, respectively, which is orders of magnitude higher than estimated savings (4) from an early-stage treatment of $0.19 and $2 per person. Similarly, the effect on caregiver burden, which accounts for a substantial proportion of societal cost (17, 18), reflected a gain $78,256 per treated individual but was negligible for an early-stage treatment (4). The reason for these substantial differences is that the cost and burden associated with the disease are already quite high at the MCI stage and, except for nursing home cost, do not change markedly by delaying progression once a patient is symptomatic (4). The difference in QALY gains is of lower relative magnitude. We estimated average gains of 0.79 and 1.85 QALYs per appropriately treated person based on an assumed treatment effectiveness of 30% and 50%, respectively, whereas published estimates for an early-stage treatment reported QALY gains of 0.75 (4), 0.73 (19), 0.65 (20), and 0.225 (21) assuming treatment effects of 30%, 25%, 31% and 25%, respectively.
While the potential value from a preventive treatment is sizeable, the result is based on using the treatment until progression to MCI and assumed annual cost of the drug $5,850 and cost could not exceed around $7,000 for the program to remain cost-effective, which is considerably lower than the list prices of aducanumab and lecanemab of around $28,200 and $26,500, respectively. However, we also show that treating for a limited time to remove amyloid deposits and monitoring for re-accumulation followed by re-treatment if needed, as successfully tested for donanemab (2), would be cost-effective up to an annual treatment cost of around $60,000. As the lower amyloid burden in preclinical individuals makes it less likely that chronic treatment at full dosing would be required, the results suggest that a secondary prevention program could generate adequate value for money over a range of assumptions. However, as others have shown before (22), economic viability depends on the interaction of effect size of the treatment and price both of which are unknown at this point. It also has to be kept in mind that the budget impact could be large because of the high prevalence of the disease, but the uptake of such a prevention program is likely to be gradual and limited. Even well-established prevention programs, such as screening for breast, cervical and colorectal cancer have a reported uptake of 81, 72 and 63 percent, respectively (23, 24, 25). Similarly, secondary prevention with PCSK9 inhibitors is regarded underutilized (26).
In addition to drug cost, performance characteristics of an AD blood test will play a critical role for the cost-effectiveness of a prevention program. As expected, generated value scales up linearly with the specificity of the test, as a lower false positive rate avoids treating individuals without the disease. In contrast, higher sensitivity decreases value and increases cost per person. This phenomenon is likely due to a cumulative false positive rate if screening tests with high sensitivity are used repeatedly, as it is known from periodic cancer screening programs (27, 28). In fact, at our base case assumptions 41% of treated individuals are false positive. The findings would suggest that a two-step screening process might be beneficial that combines a blood test with high sensitivity as first step and then a second blood test with specificity close to current confirmatory tests to make a treatment decision.
Limitations
We caution that this analysis has substantial limitations. First and foremost, it is not a true cost-effectiveness analysis because we rely on assumptions for many critical components, such as the exact nature of the clinical pathway, the administration route, frequency and cost of blood tests and/or other diagnostic assessments, test performance, treatment age range, treatment effect size, duration of treatment after Aβ plaques are removed, and durability of the effect. The predictions are very sensitive to the performance of the blood test, and population-level data for their performance remain scarce, although we use a conservative assumption of 80% accuracy. We use highly aggregated data from a prior publication to estimate the cost of a progression to MCI that may over- or underestimate the actual impact. Similarly, we assume homogenous benefits by age group. While we account for age-related mortality effects, there may be difference in benefit because of higher burden of other diseases that affect memory and cognition in older age groups. Our base case calculations assume chronic intravenous treatment, for which the two currently approved drugs (aducanumab and lecanemab) are labeled, but it is unclear whether chronic treatment at the full dose will be required. Similarly, our assumptions for time-limited treatment may prove to be incorrect. The estimates apply to intravenous treatment with antibodies targeting Aβ plaques and would not generalize to oral or subcutaneous treatments and treatments with different mechanisms of action and monitoring requirements. Lastly, we note that we specifically assessed a scenario for a secondary prevention therapy in unimpaired individuals with biomarker evidence of the AD. Different assumptions and calculations would be needed for a primary prevention therapy in unimpaired persons at genetic risk of developing AD, which would start before biomarker evidence of the disease.
Conclusions
Despite the limitations, the analysis suggests that a preventive treatment for AD could be economically viable based on a defensible set of assumptions. While those assumptions need refined further based on emerging data on blood test performance and treatment effect size, we hope that these initial projections will stimulate a discussion among stakeholders about the contours of such a prevention program, as well as the strategies to optimize affordability and accessibility within the next 2-3 years, well before findings from ongoing AD prevention trials are completed.
Funding: The project was supported by a grant to USC from Banner Alzheimer’s Foundation and a National Institute on Aging grant P30 AG072980 (Reiman, PI). Open access funding provided by SCELC, Statewide California Electronic Library Consortium.
Conflicts of interest: Dr. Mattke serves on the board of directors of Senscio Systems, Inc., and the scientific advisory board of AiCure Technologies, ALZPath and Boston Millennia Partners. He has received consulting fees from Biogen, C2N, Eisai, Novartis, and Roche/Genentech. Dr. Kordower is a consultant for Inhibikase inc, Biogen inc, Seelos inc, Amydys inc, Rocket Pharmaceuticals, MarkTx inc, The Michael J. Fox Foundation, Encora Inc, The Aufzien Center for Prevention of Parkinson and other Neurodegenerative Disease, Clintrex inc, and Exicure inc. Dr. Reiman is a co-PI of the Alzheimer’s Prevention Initiative (API), including its co-leadership role with Eli Lilly in TRAILBLAZER ALZ-3, a prevention trial of cognitively unimpaired persons with plasma pTau217 biomarker evidence of amyloid-β plaque burden; a co-founder, advisor and shareholder of ALZPath, which aims to advance the role of blood-based biomarkers in Alzheimer’s disease research, treatment development and clinical care; a compensated scientific advisor to Alzheon, Aural Analytics, Denali, Retromer Therapeutics, and Vaxxinity, and an uncompensated advisor to Biogen and Eli Lilly. The other authors report no conflicts of interest.
Consent statement: As the study did not constitute human subjects research per U.S. federal regulations (45 CFR 46, 102(f))20, it was exempt from IRB review, consent requirements and registration.
Open Access: This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
References
1. van Dyck CH, Swanson CJ, Aisen P, et al. Lecanemab in Early Alzheimer’s Disease. N Engl J Med. Nov 29 2022;doi:10.1056/NEJMoa2212948
2. Sims JR, Zimmer JA, Evans CD, et al. Donanemab in Early Symptomatic Alzheimer Disease: The TRAILBLAZER-ALZ 2 Randomized Clinical Trial. JAMA. Jul 17 2023;doi:10.1001/jama.2023.13239
3. Rafii MS, Sperling RA, Donohue MC, et al. The AHEAD 3-45 Study: Design of a prevention trial for Alzheimer’s disease. Alzheimer’s & Dementia. 2022;doi:10.1002/alz.12748
4. Prados MJ, Liu Y, Jun H, Lam J, Mattke S. Projecting the long-term societal value of a disease-modifying treatment for Alzheimer’s disease in the United States. Alzheimers Dement. Jan 2022;18(1):142-151. doi:10.1002/alz.12578
5. Robinson RL, Rentz DM, Andrews JS, et al. Costs of Early Stage Alzheimer’s Disease in the United States: Cross-Sectional Analysis of a Prospective Cohort Study (GERAS-US)1. Journal of Alzheimer’s Disease. 2020;75(2):437-450. doi:10.3233/jad-191212
6. Krist AH, Davidson KW, Mangione CM, et al. Screening for Lung Cancer. JAMA. 2021;325(10):962. doi:10.1001/jama.2021.1117
7. Davidson KW, Barry MJ, Mangione CM, et al. Screening for Colorectal Cancer. JAMA. 2021;325(19):1965. doi:10.1001/jama.2021.6238
8. Jansen WJ, Janssen O, Tijms BM, et al. Prevalence Estimates of Amyloid Abnormality Across the Alzheimer Disease Clinical Spectrum. JAMA Neurology. 2022;79(3):228. doi:10.1001/jamaneurol.2021.5216
9. Parnetti L, Chipi E, Salvadori N, D’Andrea K, Eusebi P. Prevalence and risk of progression of preclinical Alzheimer’s disease stages: a systematic review and meta-analysis. Alzheimer’s Research & Therapy. 2019;11(1)doi:10.1186/s13195-018-0459-7
10. Cho SH, Woo S, Kim C, et al. Disease progression modelling from preclinical Alzheimer’s disease (AD) to AD dementia. Scientific Reports. 2021;11(1)doi:10.1038/s41598-021-83585-3
11. Roberts RO, Aakre JA, Kremers WK, et al. Prevalence and Outcomes of Amyloid Positivity Among Persons Without Dementia in a Longitudinal, Population-Based Setting. JAMA Neurology. 2018;75(8):970. doi:10.1001/jamaneurol.2018.0629
12. Shcherbinin S, Guerguieva I, Cheng Y-J, et al. Amyloid re-accumulation after dononemab treatment: summary from 3 interventional trials. presented at: AAIC 2023; 2023; Amsterdam.
13. Coerver K, Yu MM, D’Abreu A, Wasserman M, Nair KV. Practical Considerations in the Administration of Aducanumab for the Neurologist. Neurology: Clinical Practice. 2022;12(2):169-175. doi:10.1212/cpj.0000000000001144
14. Cummings J, Aisen P, Apostolova LG, Atri A, Salloway S, Weiner M. Aducanumab: Appropriate Use Recommendations. The Journal of Prevention of Alzheimer’s Disease. 2021:1-13. doi:10.14283/jpad.2021.41
15. Strand BH, Knapskog A-B, Persson K, et al. The Loss in Expectation of Life due to Early-Onset Mild Cognitive Impairment and Early-Onset Dementia in Norway. Dementia and Geriatric Cognitive Disorders. 2019;47(4-6):355-365. doi:10.1159/000501269
16. Mooldijk SS, Yaqub A, Wolters FJ, et al. Life expectancy with and without dementia in persons with mild cognitive impairment in the community. Journal of the American Geriatrics Society. 2022;70(2):481-489. doi:10.1111/jgs.17520
17. Schaller S, Mauskopf J, Kriza C, Wahlster P, Kolominsky-Rabas PL. The main cost drivers in dementia: a systematic review. International Journal of Geriatric Psychiatry. 2015;30(2):111-129. doi:10.1002/gps.4198
18. Pyenson B, Sawhney TG, Steffens C, et al. The Real-World Medicare Costs of Alzheimer Disease: Considerations for Policy and Care. J Manag Care Spec Pharm. Jul 2019;25(7):800-809. doi:10.18553/jmcp.2019.25.7.800
19. Wimo A, Handels R, Winblad B, et al. Quantifying and Describing the Natural History and Costs of Alzheimer’s Disease and Effects of Hypothetical Interventions. J Alzheimers Dis. 2020;75(3):891-902. doi:10.3233/JAD-191055
20. Herring WL, Gould IG, Fillit H, et al. Predicted Lifetime Health Outcomes for Aducanumab in Patients with Early Alzheimer’s Disease. Neurology and Therapy. 2021;doi:10.1007/s40120-021-00273-0
21. Ito K, Chapman R, Pearson SD, Tafazzoli A, Yaffe K, Gurwitz JH. Evaluation of the Cost-effectiveness of Drug Treatment for Alzheimer Disease in a Simulation Model That Includes Caregiver and Societal Factors. JAMA Netw Open. Oct 1 2021;4(10):e2129392. doi:10.1001/jamanetworkopen.2021.29392
22. Jun H, Cho SK, Aliyev ER, Mattke S, Suen SC. How Much Value Would a Treatment for Alzheimer’s Disease Offer? Cost-Effectiveness Thresholds for Pricing a Disease-Modifying Therapy. Curr Alzheimer Res. Dec 3 2020;doi:10.2174/1567205017666201203121907
23. Ovod V, Ramsey KN, Mawuenyega KG, et al. Amyloid β concentrations and stable isotope labeling kinetics of human plasma specific to central nervous system amyloidosis. Alzheimer’s & Dementia. 2017;13(8):841-849. doi:10.1016/j.jalz.2017.06.2266
24. Petersen RC, Lopez O, Armstrong MJ, et al. Practice guideline update summary: Mild cognitive impairment: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. Jan 16 2018;90(3):126-135. doi:10.1212/WNL.0000000000004826
25. Verberk IMW, Slot RE, Verfaillie SCJ, et al. Plasma Amyloid as Prescreener for the Earliest A lzheimer Pathological Changes. Annals of Neurology. 2018;84(5):648-658. doi:10.1002/ana.25334
26. Bradley CK, Kolkailah AA, Shah NP, Page CB, Peterson ED, Navar AM. Uptake of non-statin lipid-lowering therapies for secondary prevention in community practice. J Clin Lipidol. May-Jun 2023;17(3):412-414. doi:10.1016/j.jacl.2023.03.006
27. Haug U, Coupé VMH. The cumulative false-positive rate in colorectal cancer screening: a Markov analysis. European Journal of Gastroenterology & Hepatology. 2020;32(5):575-580. doi:10.1097/meg.0000000000001669
28. Croswell JM, Kramer BS, Kreimer AR, et al. Cumulative Incidence of False-Positive Results in Repeated, Multimodal Cancer Screening. The Annals of Family Medicine. 2009;7(3):212-222. doi:10.1370/afm.942
© The Authors 2024