Y.Y. Lim1, J. Kong2, P. Maruff3,4, J. Jaeger5, E. Huang2, E. Ratti2
1. Turner Institute for Brain and Mental Health, School of Psychological Sciences, Monash University, Clayton, Australia; 2. Biogen, Cambridge, MA, USA; 3. Florey Institute of Neuroscience and Mental Health, University of Melbourne, Parkville, Australia; 4. Cogstate Ltd., Melbourne, Australia; 5. CognitionMetrics, Inc, Wilmington, Delaware and Albert Einstein College of Medicine, New York, NY, United States
Corresponding Author: Yen Ying Lim, Turner Institute for Brain and Mental Health, 18 Innovation Walk, Clayton VIC 3168, Australia, yenying.lim@monash.edu, Ph: +61 4 3387 3222
J Prev Alz Dis 2022;1(9):178-183
Published online December 1, 2021, http://dx.doi.org/10.14283/jpad.2021.64
Abstract
Sensitive cognitive assessments accurately detect and track cognitive decline in Alzheimer’s disease. The Cogstate battery was used to measure cognitive change in cognitively normal participants and in individuals with mild cognitive impairment and mild Alzheimer’s disease enrolled in the Australian Imaging, Biomarker and Lifestyle Rate of Change Substudy. Over 18 months, verbal episodic memory performance declined for mild cognitive impairment and mild Alzeheimer’s disease groups when compared to cognitively normal participants. Frequent assessments of episodic memory may facilitate early detection of cognitive decline due to Alzheimer’s disease.
Key words: Alzheimer’s disease, mild cognitive impairment, Cogstate Brief Battery, International Shopping List Test, cognitive decline.
Introduction
Pathological changes that characterize Alzheimer’s disease (AD) (i.e., accumulation of cerebral amyloid-β [Aβ] and tau), are evident up to 20 years before dementia is classified clinically (1, 2). These changes often remain clinically silent for many years, although recently, by measuring at-risk individuals repeatedly, subtle but measurable decline in cognition—particularly in episodic and working memory—can be detected in older adults who have abnormal levels of Aβ (Aβ+) but are clinically normal (3, 4). Similarly, in individuals with mild cognitive impairment (MCI), Aβ+ is associated with cognitive decline over 3 years, whilst Aβ- individuals with MCI show impaired but stable cognition over the same period (5, 6). Cognitive decline may thus serve as one of the earliest detectable manifestations of an underlying AD pathophysiology. Additionally, detection of cognitive dysfunction may be more sensitive when based on the repeated assessment over time than on the basis of a single assessment (7, 8).
One challenge in the detection of cognitive decline in the earliest stages of AD is that neuropsychological tests of memory and working memory have not been designed to be applied repeatedly, especially over relatively short re-test intervals (e.g., months) (9, 10). Additionally, for those that are, the number of parallel forms is often limited. The repeated application of such tests can lead to substantial improvements in performance (i.e., practice effects), even in individuals with memory impairment severe enough to warrant classification as MCI or dementia (11, 12).
The Cogstate Brief Battery (CBB) and the International Shopping List Test (ISLT) were developed and validated specifically with the intent of repeated application over very short re-test intervals (e.g., hours, days) in both cognitively normal (CN) older adults and in adults with dementia (5, 13). The visual learning and working memory tests from the Cogstate battery as well as the ISLT have been shown to be sensitive to cognitive decline in both preclinical (Aβ+ CN) and prodromal (Aβ+ MCI) AD, albeit with long retest intervals (e.g., 18 months) over study periods of five to seven years (14, 15). This general sensitivity to cognitive decline in Aβ+ individuals raises the possibility that more frequent re-testing (e.g., at three month intervals or less) could allow detection of AD-related cognitive decline in prodromal and clinical AD over short time periods (e.g., months) even in small samples (e.g., <50). The Australian Imaging, Biomarkers and Lifestyle (AIBL)-Rate of Change Sub-Study (i.e., AIBL-ROCS) was designed to test this hypothesis in Aβ+ MCI and mild AD groups relative to Aβ- CN older adults.
Methods
Participants
Analyses were conducted on longitudinal data collected from the AIBL-ROCS cohort. Detailed inclusion and exclusion criteria for AIBL-ROCS have been described previously (16). Briefly, participants aged 60-96 were recruited, with a consensus classification by the AIBL clinical panel as either CN, or having amnestic MCI or AD dementia, according to Winblad 2004 guidelines and NINCDS-ADRDA criteria respectively (17). Inclusion in AIBL-ROCS was contingent upon the ability to perform computerized cognitive tasks, and a willingness to undergo more frequent visits to allow for high-frequency serial cognitive assessments. All patients with AD dementia were receiving treatment with acetylcholinesterase medications and/or memantine. The AIBL study was approved by the institutional ethics committees of Austin Health (Victoria), St. Vincent’s Hospital (Victoria), Hollywood Private Hospital (Western Australia), and Edith Cowan University (Western Australia). All CN and MCI participants provided written informed consent before participation. Written informed consent was obtained from the carers of all participants with AD dementia.
Study Design, Measures, and Procedures
Aβ+ was defined as a positron emission tomography (PET) standardized uptake value ratio (SUVR) >1.5 using Pittsburgh compound B (PiB). Following a practice session, cognitive assessment with the computerized CBB was conducted at baseline and at 3, 6, 9, 12, 15, and 18-month follow-up (16). A trained assessor was assigned to conduct repeated assessments and organize home visits with each participant. Study visit times were held constant by raters, with up to one-week variation in follow-up assessments. This analysis focused on 6 tests from the CBB, all of which have been described in detail previously (16, 18). Briefly, the Detection (DET) test was a measure of simple reaction time, the Identification (IDN) test was a measure of visual attention via choice reaction time, the One Card Learning (OCL) test was a measure of visual learning and memory set within a pattern separation framework, the One-Back (OBK) test was a measure of working memory, and the ISLT was a verbal list learning test of episodic memory, with two outcomes (i.e., total words recalled over (a) three learning trials, and (b) 30-minute delayed recall). Participants also completed the AIBL clinical and neuropsychological assessment battery, which included the Mini-Mental State Examination (MMSE), the Clinical Dementia Rating (CDR) scale, the California Verbal Learning Test, second edition (CVLT-II), the Stroop test, and the Hospital Anxiety and Depression Scale. The AIBL battery was administered at 18-month intervals.
Statistical Analyses
All analyses were conducted in R v.3.4.2, using the packages “psych”, “gmodels”, “lmerTest”, and “lme4”. Participants were classified as Aβ− CN, Aβ+ MCI, or Aβ+ AD. For each Cogstate outcome measure, longitudinal change over 18 months was assessed using linear mixed models (LMM) with an unstructured covariance matrix, with participants and time as random factors. Time was treated as a continuous variable. In considering the potential benefits of repeated cognitive assessments, we also examined longitudinal change of each group on the CVLT-II total and delayed recall scores, assessed at baseline and 18 months, using LMMs. Statistical significance was set at p<.05, and no corrections for multiple comparisons were made. However, to minimize the potential for conclusions based on Type I error, we also computed Cohen’s d effect sizes to contextualize the magnitude of cognitive decline between groups.
Results
Participant Enrollment
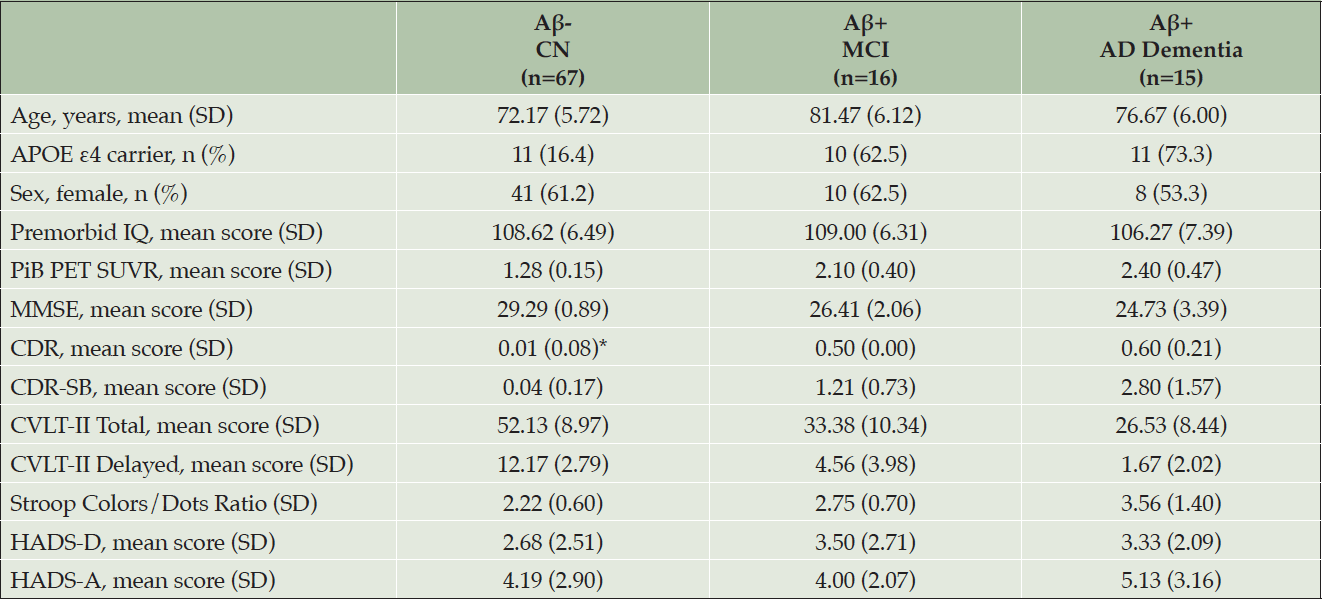
Of the 205 participants enrolled in AIBL-ROCS, a subgroup underwent Aβ PET neuroimaging (Table 1), and 90 participants completed the 18-month study. At baseline, mean CDR-Sum of Boxes (CDR-SB) in the Aβ+ AD group was consistent with the classification of mild dementia (Table 1). The median number of assessments for all groups was 7.
* A participant with CDR score of 0.5 was classified as cognitively normal if they 1) did not meet the Winblad criteria or Petersen criteria for MCI and 2) performed within age- and education-based limits in neuropsychological testing. CDR ratings were informed by neuropsychological testing and not rated independently; APOE, apolipoprotein E; CDR, Clinical Dementia Rating; CDR-SB, Clinical Dementia Rating Scale-Sum of Boxes; CVLT-II, California Verbal Learning Test, second edition; HADS-A, Hospital Anxiety and Depression Scale-Anxiety subscale; HADS-D, Hospital Anxiety and Depression Scale-Depression subscale; MMSE, Mini-Mental State Examination; PET, positron emission tomography; PiB, Pittsburgh compound B; SD, standard deviation; SUVR, standardized uptake value ratio
Longitudinal Decline Within Groups
Both Aβ+ MCI and Aβ+ mild AD groups showed significant (p<.05) decline in the ISLT total score over 15 and 18 months respectively (Figure 1A). Significant decline on the ISLT delayed score was also observed in the Aβ+ MCI group over 1 -months but not in the Aβ+ mild AD group (Figure 1B). The Aβ+ MCI and Aβ+ mild AD groups did not show any significant decline on any other cognitive measure (Supplement Figure 1). No significant decline on the CVLT-II total (β (SE)=-0.038 (0.059), p=.525) or delayed (β (SE)=-0.053 (0.078), p=.499) recall was observed in Aβ+ MCI over 18 months. Aβ+ mild AD showed significant decline on the CVLT-II total (β (SE)=-0.138 (0.061), p=.026), but not delayed (β (SE)=-0.114 (0.081), p=.163) recall over 18 months.
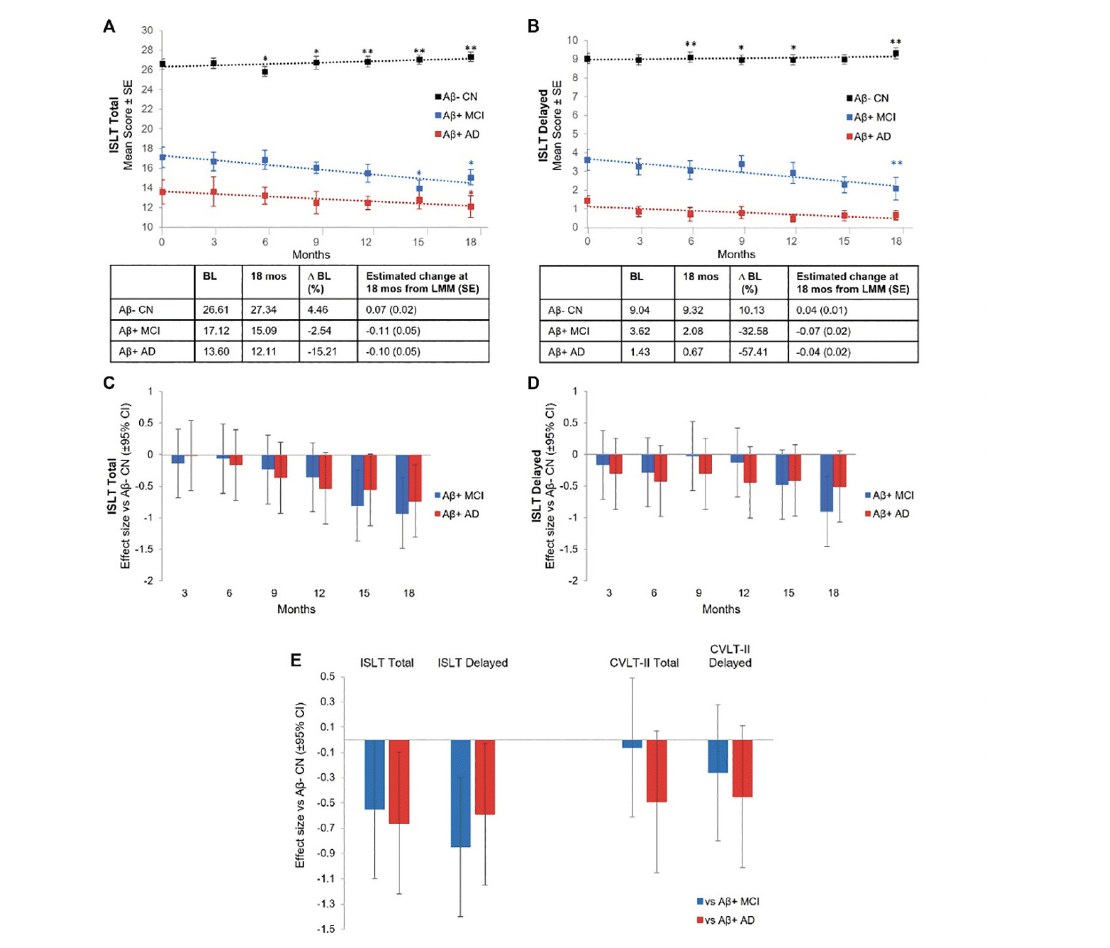
Figure 1. Longitudinal Change and Effect Size vs Aβ- CN Controls for ISLT Total and ISLT Delayed and Effect Size vs Aβ- CN Controls for CVLT-II Total and CVLT-II Delayed
ISLT Total (A) and ISLT Delayed (B) mean scores for Aβ- CN (Black Line), Aβ+ MCI (Blue Line), and Aβ+ AD (Red Line), effect sizes for Aβ+ MCI (Blue) and Aβ+ AD (Red) versus Aβ- CN on ISLT Total (C) and ISLT Delayed (D) at 3-month intervals over 18 months, and effect sizes for Aβ+ MCI (Blue) and Aβ+ AD (Red) versus Aβ- CN on ISLT Total, ISLT Delayed, CVLT-II Total, and CVLT Delayed at 18 months (E); * p<.05, † p<.01 LMM slope significantly different from baseline; Aβ, amyloid-β; AD, Alzheimer’s disease; BL, baseline; CI, confidence interval; CN, cognitively normal; CVLT-II, California Verbal Learning Test, second edition; ISLT, International Shopping List Test; LMM, linear mixed model; MCI, mild cognitive impairment; Mos, months; SE, standard error.
For the Aβ- CN group, no significant decline in any cognitive outcome was observed over the 18-month re-test period (Figure 1A and B, Supplement Figure 1). Similarly, no significant change was observed in the CVLT-II total (β (SE)=-0.023 (0.028), p=.416) or delayed (β (SE)=0.026 (0.038), p=.491) recall scores over 18 months. However, significant improvement in performance from baseline was observed for the Aβ− CN group for the measures of ISLT total, ISLT delayed, and OCL.
Comparison of Rate of Cognitive Decline Between CN Older Adults and Symptomatic Groups
Compared to the Aβ- CN group, the Aβ+ MCI group showed a significantly faster rate of decline on the ISLT total recall score at 15 months onwards (Figure 1C; Aβ+ MCI vs Aβ− CN d=0.81, p=.004 at 15 months; Aβ+ MCI vs Aβ− CN d=0.93, p=.001 at 18 months), and on the ISLT delayed recall score at 18 months (Figure 1D, Aβ+ MCI vs Aβ− CN d=0.91, p=.002). Compared to Aβ- CNs, the Aβ+ mild AD group showed a significantly greater rate of decline over 18 months only on the ISLT total recall score (Figure 1C, Aβ+ AD vs Aβ− CN d=0.74, p=.01), but not on the ISLT delayed recall score (Figure 1D, Aβ+ AD vs Aβ− CN d=0.51, p=.08). Over the 18-month re-test period, Aβ+ MCI and Aβ+ mild AD groups did not show significant decline on any other cognitive measure when compared with Aβ- CNs (Supplement Figure 1). Similarly, Aβ+ MCI and Aβ+ mild AD groups did not show significant rates of decline on the CVLT-II total (MCI d=0.07, p=.828; AD d=0.50, p=.093), and delayed (MCI d=0.25, p=.363; AD d=0.45, p=.120) recall scores when compared with Aβ- CNs (Fig 1E). When data on the ISLT were restricted to two timepoints to match those upon which the CVLT-II was administered (i.e., baseline and 18 months), compared to the Aβ- CN group, the Aβ+ MCI group had a significantly faster rate of decline over the 18 months for the ISLT total recall (β (SE)=-0.144 (0.078), d=0.55), p=.051), and ISLT delayed recall (β (SE)=-0.213 (0.075), d=0.85), p=.005) scores (Fig 1E). The same comparisons for the Aβ+ mild AD group also showed a significantly faster rate of decline at the 18-month timepoint on the ISLT total recall (β (SE)=-0.174 (0.084), d=0.66), p=.024), and the ISLT delayed recall (β (SE)=-0.147 (0.077), d=0.59), p=.044) scores (Fig 1E).
Discussion
In this study, Aβ+ MCI and Aβ+ mild AD groups showed longitudinal decline in episodic memory over the 15-18 months of assessment. Specifically, when assessed seven times across the 18-month study period, the Aβ- CN group showed no loss of words on the ISLT total or delayed recall score. Conversely, compared to the Aβ- CN group, the Aβ+ MCI group showed a faster rate of memory decline over the same interval on the ISLT total and delayed recall scores (d=1) (Fig 1). The Aβ+ mild AD group also demonstrated a faster rate of memory decline over the 18-month test–re-test interval when compared to the Aβ- CN group, but only on the ISLT total recall score (d=0.74), and not on the delayed recall score (d=0.51) (Fig 1). On the ISLT delayed recall score, the Aβ+ mild AD group performed at a stable, but very impaired, level of ~8 words below the Aβ- CN group. It is likely that the smaller magnitude of decline on the ISLT delayed recall score in the Aβ+ mild AD group is because this group’s delayed recall performance was already at or near the lowest possible score (e.g., 0 or 1) at baseline. Thus, while the ISLT delayed recall score may serve as a useful screening tool at baseline to identify patients with clinically significant cognitive impairment, its utility in measuring change over time, particularly in those who have progressed to the dementia stages of AD, may be limited.
In contrast to the measure of verbal memory, no decline was observed for measures of processing speed and attention in either Aβ+ MCI or Aβ+ mild AD groups, and the effect sizes for differences in slopes for these outcomes were small (e.g., <1% change from baseline). The absence of any decline in processing speed and attention in this study is consistent with observations from previous studies [5, 19, 20], and confirms that cognitive decline in AD does not manifest in simple or reflexive aspects of cognition. Compared to Aβ- CNs, no decline in visual memory or working memory was observed in the Aβ+ MCI or Aβ+ mild AD groups, although effect sizes for some comparisons indicated that for both groups, the rate of cognitive decline was moderate in magnitude (Supplement Figure 1). Statistically significant decline in visual memory and working memory have been observed previously in the Aβ+ MCI and Aβ+ mild AD groups from the broader AIBL cohort, albeit studied at longer re-test intervals, for example, over 18 and 36 months. It is possible that practice effects resulting from the many repeated assessments given in this study acted to obscure any true decline in cognitive function. However, assessments of memory at the beginning and end of the 18-month study period with the CVLT-II total and delayed recall scores also showed no statistically significant decline. Additionally, while previous studies of the AIBL cohort have used sample sizes greater than those studied here, the effect sizes observed were similar in magnitude (5, 21). Thus, the absence of statistically significant decline in visual memory and working memory more likely occurred because the relatively small sample size did not provide statistical power adequate to render the moderate magnitude of decline statistically significant, despite the multiple assessments. Thus, while the high frequency repeated assessment does allow detection of AD-related cognitive decline over intervals of approximately 18 months, the magnitude of decline detected here indicates that larger sample sizes will be needed to render such decline statistically significant.
No statistically significant decline over 18 months was observed in Aβ- CN older adults on any cognitive test. While this is consistent with previous work showing the absence of cognitive decline in Aβ- CNs using the same battery over 36 months (14), we did observe modest improvements in performance on the ISLT, OBK, and OCL tests. It is possible that practice effects from the 6 reassessments within the 18 months resulted in these slight improvements. Our observation that these improvements occur primarily in tests of episodic and working memory are consistent with previous studies that have similarly demonstrated that Aβ- CNs can benefit from repeated exposure to episodic memory tests, while those with underlying AD pathology do not (22-25).
A key limitation of this study is that the sample size of the Aβ+ MCI and Aβ+ mild AD groups was very small (i.e., <20). Despite this, the decline in episodic memory detected using the ISLT was sufficient to reach statistical significance in the prodromal and mild AD stages. As a general principle of measurement, the ability of a measure to detect change in cognitive function can increase with the number of measurements used to estimate that change by offering more precise slope estimates and improving the detection of the decline signal over the statistical noise arising from normal day to day variance in cognitive performance. This is supported by the observation that no statistically significant decline was observed for the CVLT-II total and delayed recall scores in the same groups when assessed only at baseline and 18 months (e.g., 11% change from baseline on the CVLT-II total recall score vs. 33% change from baseline on the ISLT total recall score). When data on the ISLT were restricted to baseline and 18 months, we observed a statistically significant decline on both the ISLT total and delayed recall scores that was of a moderate magnitude. However, this greater magnitude of decline at 18 months on the ISLT total and delayed recall scores (Fig 1E) may include signal from the repeated assessments that may not be explicit in the analysis.
These limitations notwithstanding, in high-risk populations, quarterly measurement may improve clinical decision making regarding the presence of cognitive decline. To better understand the longitudinal sensitivity of the Cogstate battery with various risk groups, future investigations with larger sample sizes and longer follow up will provide additional power to examine potentially informative covariances (e.g., APOE ε4). The ISLT has been and is currently used in clinical trials as a primary or secondary endpoint for monitoring the progression of AD and other dementias (NCT03402503, NCT01009255, NCT02244541, NCT02579252, NCT01760005, NCT03088956). As frequent re-assessments of the ISLT and the Cogstate battery may reduce the need for large sample sizes of at-risk individuals, this analysis supports further investigation of these tests as cognitive endpoints in clinical trials seeking to halt or slow cognitive decline.
Acknowledgements: The AIBL-ROCS study thanks the participants and their families for their involvement in the study.
Funding: Funding for AIBL-ROCS was provided by AstraZeneca Pharmaceuticals LP. Funding for the AIBL study was provided in part by the study partners (Australian Commonwealth Scientific Industrial and Research Organisation, Edith Cowan University, Mental Health Research Institute, Alzheimer’s Australia, National Ageing Research Institute, Austin Health, Cogstate Ltd., Hollywood Private Hospital, and Sir Charles Gardner Hospital). This analysis was funded by Biogen. Writing and editorial support, under direction of the authors, was provided by Nucleus Global and was funded by Biogen. Biogen was not directly involved in data analysis. However, Biogen identified the research question, participated in the interpretation of the results, and in the writing and reviewing of the manuscript. YY Lim is supported by an NHMRC Career Development Fellowship (GNT1162645).
Conflict of Interest: Dr. Lim has nothing to disclose. Dr. Huang has received personal compensation for consulting, serving on a scientific advisory board, speaking, or other activities with Biogen and has received personal compensation in an editorial capacity for Biogen. Dr. Kong has received personal compensation for consulting, serving on a scientific advisory board, speaking, or other activities with Biogen and holds stock and/or stock options in Biogen. Dr. Maruff has received personal compensation for consulting, serving on a scientific advisory board, speaking, or other activities with Cogstate. Dr. Jaeger is an employee and owner of CognitionMetrics, LLC, which provides scientific consulting services to Biogen. Dr. Ratti has received personal compensation for consulting, serving on a scientific advisory board, speaking, or other activities with Biogen and holds stock and/or stock options in Biogen.
Open Access: This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
References
1. Villemagne VL, Pike KE, Chételat G, Ellis KA, Mulligan RS, Bourgeat P, Ackermann U, Jones G, Szoeke C, Salvado O, Martins R, O’Keefe G, Mathis CA, Klunk WE, Ames D, Masters CL, Rowe CC. Longitudinal assessment of Aβ and cognition in aging and Alzheimer disease. Annals of Neurology 2011;69, 181-192; doi: 10.1002/ana.22248.
2. Betthauser TJ, Koscik RL, Jonaitis EM, Allison SL, Cody KA, Erickson CM, Rowley HA, Stone CK, Mueller KD, Clark LR, Carlsson CM, Chin NA, Asthana S, Christian BT, Johnson SC. Amyloid and tau imaging biomarkers explain cognitive decline from late middle-age. Brain 2020;143, 320-335; doi: 10.1093/brain/awz378.
3. Baker JE, Lim YY, Pietrzak RH, Hassenstab J, Snyder PJ, Masters CL, Maruff P. Cognitive impairment and decline in cognitively normal older adults with high amyloid-β: A meta-analysis. Alzheimer’s & Dementia: Diagnosis, Assessment & Disease Monitoring 2016;6, 108-121; doi: 10.1016/j.dadm.2016.09.002.
4. Hedden T, Oh H, Younger AP, Patel TA. Meta-analysis of amyloid-cognition relations in cognitively normal older adults. Neurology 2013;80, 1341-1348; doi: 10.1212/WNL.0b013e31828ab35d.
5. Lim YY, Maruff P, Pietrzak RH, Ellis KA, Darby D, Ames D, Harrington K, Martins RN, Masters CL, Szoeke C, Savage G, Villemagne VL, Rowe CC, AIBL RG. Aβ and cognitive change: Examining the preclinical and prodromal stages of Alzheimer’s disease. Alzheimer’s & Dementia 2014;10, 743-751; Doi: 10.1016/j.jalz.2013.11.005.
6. Lim YY, Ellis KA, Harrington K, Kamer A, Pietrzak RH, Bush AI, Darby D, Martins RN, Masters CL, Rowe CC, Savage G, Szoeke C, Villemagne VL, Ames D, Maruff P, AIBL RG. Cognitive consequences of high Aβ amyloid in mild cognitive impairment and healthy older adults: Implications for early detection of Alzheimer’s disease. Neuropsychology 2013;27, 322-332; doi: 10.1037/a0032321.
7. Ployhart RE, Vandenberg RJ (2010) Longitudinal research: The theory, design and analysis of change. Journal of Management 2010;38, 94-120; doi: 10.1177/0149206309352110.
8. Willett JB. Some results on reliability for the longitudinal measurement of change: Implications for the design of studies of individual growth. Educational and Psychological Measurement 1989;49, 587-602; doi: 10.1177/001316448904900309.
9. Benedict RHB, Zgaljardic DJ. Practice effects during repeated administration of memory tests with and without alternate forms. Journal of Clinical and Experimental Neuropsychology 1998;20, 339-352; doi: 10.1076/jcen.20.3.339.822.
10. Collie A, Maruff P, Darby DG, McStephen M. The effects of practice on the cognitive test performance of neurologically normal individuals assessed at brief test-retest intervals. Journal of the International Neuropsychological Society 2003;9, 419-428; DOI: 10.1017/S1355617703930074.
11. Duff K, Atkinson TJ, Suhrie KR, Dalley BCA, Schaefer SY, Hammers DB. Short-term practice effects in mild cognitive impairment: Evaluating different methods of change. Journal of Clinical and Experimental Neuropsychology 2017;9, 396-407; doi: 10.1080/13803395.2016.1230596.
12. Calamia M, Markon K, Tranel D. Scoring higher the second time around: meta-analyses of practice effects in neuropsychological assessment. The Clinical Neuropsychologist 2012;26, 543-570; doi: 10.1080/13854046.2012.680913.
13. Lim YY, Villemagne VL, Laws SM, Pietrzak RH, Ames D, Fowler C, Rainey-Smith S, Snyder PJ, Bourgeat P, Martins RN, Salvado O, Rowe CC, Masters CL, Maruff P. Performance on the Cogstate Brief Battery is related to amyloid levels and hippocampal volume in very mild dementia. J Mol Neurosci 2016;60, 362-370; doi: 10.1007/s12031-016-0822-8.
14. Lim YY, Pietrzak RH, Bourgeat P, Ames D, Ellis KA, Rembach A, Harrington K, Salvado O, Martins RN, Snyder PJ, Masters CL, Rowe CC, Villemagne VL, Maruff P. Relationships between performance on the Cogstate Brief Battery, neurodegeneration, and Aβ accumulation in cognitively normal older adults and adults with MCI. Archives of Clinical Neuropsychology 2015;30, 49-58; Doi: 10.1093/arclin/acu068.
15. Pietrzak RH, Lim YY, Ames D, Harrington K, Restrepo C, Martins RN, Rembach A, Laws SM, Masters CL, Villemagne VL, Rowe CC, Maruff P. Trajectories of memory decline in preclinical Alzheimer’s disease: results from the Australian Imaging, Biomarkers and Lifestyle Flagship Study of ageing. Neurobiol Aging 2015;36, 1231-1238; doi: 10.1016/j.neurobiolaging.2014.12.015.
16. Lim YY, Jaeger J, Harrington K, Ashwood T, Ellis KA, Stöffler A, Szoeke C, Lachovitzki R, Martins RN, Savage G, Villemagne V, Bush A, Masters CL, Rowe CC, Ames D, Darby D, Maruff P. Three-month stability of the CogState Brief Battery in healthy older adults, mild cognitive impairment, and Alzheimer’s disease: Results from the Australian Imaging, Biomarkers, and Lifestyle-Rate of Change Substudy (AIBL-ROCS). Archives of Clinical Neuropsychology 2013;28, 320-330; doi: 10.1093/arclin/act021.
17. Ellis KA, Bush AI, Darby D, De Fazio D, Foster J, Hudson P, Lautenschlager NT, Lenzo N, Martins RN, Maruff P, Masters C, Milner A, Pike K, Rowe C, Savage G, Szoeke C, Taddei K, Villemagne V, Woodward M, Ames D, Group TAR. The Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging: Methodology and baseline characteristics of 1112 individuals recruited for a longitudinal study of Alzheimer’s disease. International Psychogeriatrics 2009;21, 672-687; doi: 10.1017/S1041610209009405.
18. Lim YY, Pietrzak RH, Snyder PJ, Darby D, Maruff P. Preliminary data on the effect of culture on the assessment of Alzheimer’s disease related verbal memory impairment with the International Shopping List Test (ISLT). Archives of Clinical Neuropsychology 2012;27, 136-147; doi: 10.1093/arclin/acr102.
19. Lim YY, Pietrzak RH, Ellis KA, Jaeger J, Harrington K, Ashwood T, Szoeke C, Martins RN, Bush AI, Masters CL, Rowe CC, Villemagne VL, Ames D, Darby D, Maruff P. Rapid decline in episodic memory in healthy older adults with high amyloid-β. Journal of Alzheimer’s Disease 2013;33, 675-679; doi: 10.3233/JAD-2012-121516.
20. Maruff P, Lim YY, Darby D, Ellis KA, Pietrzak RH, Snyder PJ, Bush AI, Szoeke C, Schembri A, Ames D, Masters CL, AIBL RG. Clinical utility of the Cogstate Brief Battery in identifying cognitive impairment in mild cognitive impairment and Alzheimer’s disease. BMC Pharmacology & Toxicology 2013;1, 1-11; doi: 10.1186/2050-7283-1-30.
21. Lim YY, Maruff P, Pietrzak RH, Ames D, Ellis KA, Harrington K, Lautenschlager NT, Szoeke C, Martins RN, Masters CL, Villemagne VL, Rowe CC, AIBL RG. Effect of amyloid on memory and non-memory decline from preclinical to clinical Alzheimer’s disease. Brain 2014;137, 221-231; doi: 10.1093/brain/awt286.
22. Duff K, Hammers DB, Dalley BCA, Suhrie KR, Atkinson TJ, Rasmussen KM, Horn KP, Beardmore BE, Burrell LD, Foster NL, Hoffman JM. Short-term practice effects and amyloid deposition: Providing information above and beyond baseline cognition. Journal of Prevention of Alzheimer’s Disease 2017;4, 87-92; doi: 10.14283/jpad.2017.9.
23. Hassenstab J, Ruvolo D, Jasielec M, Xiong C, Grant E, Morris JC. Absence of practice effects in preclinical Alzheimer’s disease. Neuropsychology 2015;29, 940-948; doi: 10.1037/neu0000208.
24. Jutten RJ, Grandoit E, Foldi NS, Sikkes SAM, Jones RN, Choi SE, Lamar ML, Louden DKN, Rich J, Tommet D, Crane PK, Rabin LA. Lower practice effects as a marker of cognitive performance and dementia risk: A literature review. Alzheimer’s & Dementia: Diagnosis, Assessment & Disease Monitoring 2020;12, e12055; doi: 10.1002/dad2.12055.
25. Lim YY, Baker JE, Bruns Jr L, Mills A, Fowler C, Fripp J, Rainey-Smith SR, Ames D, Masters CL, Maruff P. Association of deficits in short-term learning and Aβ and hippocampal volume in cognitively normal adults. Neurology 2020;95, 2577-2585; doi: 10.1212/WNL.0000000000010728.