K. Wang1, H. Liu1
1. Center of Medical Reproduction, the First Affiliated Hospital of Chongqing Medical University, Chongqing, China. Kanran Wang’s ORCID ID is 0000-0002-6958-7677
Corresponding Author: Hong Liu, MD, PhD, Center of Medical Reproduction, the First Affiliated Hospital of Chongqing Medical University, No.1 Youyi Street, Yuzhong District, Chongqing, China, 400016, 3367009927@qq.com
J Prev Alz Dis 2021;4(8):442-447
Published online June 24, 2021, http://dx.doi.org/10.14283/jpad.2021.35
Abstract
BACKGROUND: This study aimed to assess the relation of early-onset type 2 diabetes (age<55years) versus later in life to the risk of dementia, Alzheimer Disease (AD) dementia and stroke.
Methods: This study was based on the Framingham Heart Study Offspring cohort (FHS-OS) which is a community-based prospective cohort. Glycemic status was ascertained at serial examinations over six decades among participants who initially did not have diabetes. Surveillance for incident events including dementia and stroke has been continued for approximately 30 years.
Results: At baseline, there were 142 (5%) subjects with onset of diabetes prior to age 55 years, 172 (6%) subjects with 55-64 years, 349 (11%) subjects over 65 years and 2389 (78%) subjects without diabetes. The risk of dementia, AD and stroke increased with decreasing age of diabetes onset (P<0.05, for trend). Compared with never developing diabetes, early-onset diabetes conferred a higher risk of all dementia, AD dementia and stroke [HR 2.86(1.16-5.51) for dementia; HR 2.42(1.63-4.33) for AD; HR 2.85(1.37-3.98) for stroke]. Whereas later-onset diabetes was only associated with greater risk for stroke, neither dementia nor AD.
Conclusion: Early-onset diabetes was stronger associated with an increased risk of all dementia, AD dementia and stroke than later-onset.
Key words: Early-onset diabetes, dementia, Alzheimer disease, risk factor, Framingham Heart Study.
Introduction
Dementia is a major public health concern posing substantial burden on patients, their proxies, and national health-care systems (1-3). It comprises Alzheimer disease (AD), which contributes to 50–70% of dementia cases, vascular dementia (VD), which contributes to ~25%, and other forms of dementia[4]. The causes of dementia, especially Alzheimer’s disease (AD), remain unclear, and there are no disease-modifying therapies (5). Thus, there is an urgent need to identify factors that can prevent development of dementia to decrease the burden of this disease.
Type 2 diabetes (herein referred to as “diabetes”) is highly prevalent and manifests frequently at a younger age as well as at older age (6). Although it is known that diabetes confers substantial risk for dementia and AD, it remains unclear whether this risk significantly varies by age of diabetes onset (7). On the one hand, dementia or AD risk may be more pronounced in earlier-onset diabetes, among persons with longer durations of exposure and more likely poor glycemic control; on the other hand, the risk may be greater in later-onset diabetes, among persons in whom age-related risk factors for dementia tend to aggregate (8). Besides, earlier-onset diabetes may represent a more aggressive form of disease, characterized by a much more rapid deterioration of the β-cell function with a more frequent need for insulin therapy and rapidly raised the incidence of macrovascular and microvascular complications (9, 10). Furthermore, plenty of large-scale epidemiological studies have confirmed that early-onset diabetes is associated with greater risk for adverse outcomes including mortality, cardiovascular disease, metabolic disease and psychological disease (11). However, to our knowledge, few number of epidemiological studies have assessed the relationship between early-onset diabetes and dementia and AD. At the same time, we intended to explore the relationship between early-onset diabetes and the risk for stroke in the analysis, as both stroke and dementia share common risk factors and etiologies.
Accordingly, using large-scale data from community based Framingham Heart Study Offspring cohort (FHS-OS) with detailed review of all medical records and a nearly 30-year follow-up, we aimed to examine the long-term dementia, AD and stroke risk associated with developing diabetes early versus late in the adult life course.
Methods
Study Design
This study was carried out as a secondary analysis of data from the population-based Framingham Heart Study Offspring cohort (FHS-OS) (12). The FHS-OS is a longitudinal community-based study established in 1971 and includes 5124 men and women who were children and spouses of children of the original Framingham Heart Study (FHS). And the participants of FHS-OS were reassessed 8 years after the baseline examination (in 1971) and every 4 years thereafter including standardized interviews, physician examinations, and laboratory testing. The details of the study design of FHS-OS have previously been described elsewhere (13). This study complied with the Declaration of Helsinki. The Boston Medical Center’s institutional review board approved all study protocols, and all participants provided informed consent. The National Heart, Lung, and Blood Institute (NHLBI) of National Institutes of Health (NIH) has approved this study protocol as well.
Type 2 Diabetes Assessment
Non fasting blood glucose was assessed at the first two examinations; in the latter cohort, fasting blood glucose was assessed at all subsequent examinations. Diabetes status was defined as presence of fasting blood glucose>126mg/dL or non-fasting blood glucose >200 mg/dL, or self-reported use of insulin or oral hypoglycemic agents, at two consecutive examinations (to ensure the stability over time for a given glycemic phenotype) (14).We defined disease onset as the first examination at which the criteria for diabetes was met, which was ascertained with use of all available plasma glucose data collected at serial examinations attended by FHS-OS participants. What’s more, we assumed that diagnosed diabetes was type 2 diabetes based on extremely low rates of type 1 diabetes in our cohort and the high prevalence of type 2 diabetes in the U.S (15). The “early-onset” diabetes was defined as diabetes diagnosed prior to age 55 years, given epidemiological data suggesting characteristics including clinical feature, morbidity, mortality and healthcare expenditure among persons with diabetes similar to characteristics among this age-group (16, 17). We also selected age 55 years as the threshold for defining early-onset diabetes to optimize the number of individuals and, in turn, statistical power for analyzing individuals in our study who had suffered the dementia, AD dementia or stroke prior to reaching this age threshold.
Ascertainment of Dementia and AD
The surveillance methods and dementia tracking for the FHS-OS have been published (see supplement eMethods for details). Cognitive screening is performed at each FHS-OS examination cycle using the Mini-Mental State Examination (MMSE) supplemented with extensive neuropsychological testing at selected examination cycles. A diagnosis of dementia was made in accordance with the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (18). And Alzheimer’s disease (AD) based on the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s disease and Related Disorders Association (NINCDS-ADRDA) for definite, probable, or possible AD (19).
Ascertainment of stroke
Stroke incidence was assessed through the continuous monitoring of hospital admissions in Framingham and by reviewing all available medical records and results. Stroke was defined as focal neurological symptoms of rapid onset and presumed vascular origin, lasting >24 hours or resulting in death within 24 hours. A committee comprising of least 3 FHS investigators, including at least 2 neurologists, adjudicated stroke diagnosis. The committee considered all available medical records, brain imaging, cerebrovascular imaging, and the assessment of the study neurologist who visited the participant (20).
Covariates
The clinical covariates were drawn from the first examination cycle at which data were available. BMI was calculated as kg/m2. Waist circumference (in inches) was measured at the level of the umbilicus. Current smokers were defined as participants who smoked regularly in the year preceding the examination cycle. The educational level and use of antihypertensive medications were assessed by medical interview. Total cholesterol, low-density lipoprotein cholesterol and fasting blood glucose were measured after an overnight (>10 hours) fast.
Statistical Analysis
Descriptive statistics was performed for the 4 subgroups: participants with age at onset of diabetes less than 55 years, 55-64 years and over 65 years, and persons without ever developing diabetes serving as the referent group. For continuous variables mean, standard deviation and range were calculated for approximately normally distributed data, otherwise median and range were used. For discrete data, absolute and relative frequencies were computed. Follow-up for dementia and stroke was from the baseline examination to the time of incident event. And for persons with no incident events, follow-up was censored at the time of death or the date the participant was last known to be dementia or stroke free. For survival analysis, we used multivariable Cox regression to relate case-versus-control status to age-group at onset of diabetes with adjustment for age at dementia, sex, smoking status, body mass index (BMI), systolic blood pressure (SBP), education levels, serum total cholesterol, use of antihypertensive therapy, MMSE and duration of diabetes. Hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated. The same analysis was carried out for Alzheimer’s disease and stroke. All analyses were performed with SAS software, version 9.4 (SAS Institute, Cary, NC). A 2-sided with P<0.05 was considered statistically significant.
Results
Subjects
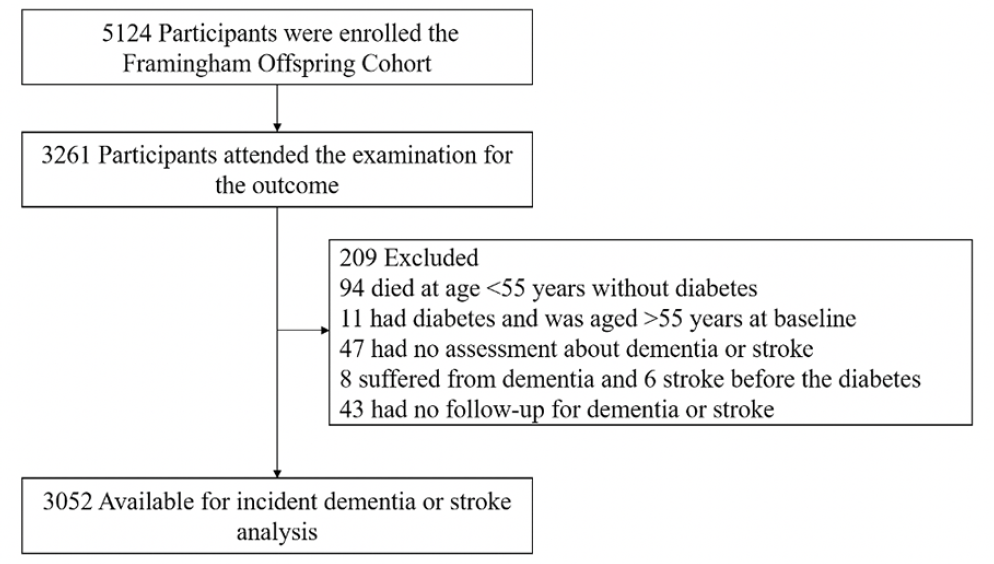
Of the 5142 participants of the FHS-OS, 3261 subjects attend the last examination for outcome ascertainment. Wherein, 209 subjects were excluded because 94 died at age <55 years without diabetes, 11 had diabetes and was aged >55 years at baseline, 47 had no assessment about dementia or stroke, 8 suffered from dementia and 6 stroke before the diabetes and 43 had no follow-up for dementia or stroke (Figure 1). Thus, 3052 subjects could be included in the final analyses, in which 142 (5%) subjects with onset of diabetes prior to age 55 years, 172 (6%) subjects with 55-64 years, 349 (11%) subjects over 65 years and 2389 (78%) subjects without diabetes.
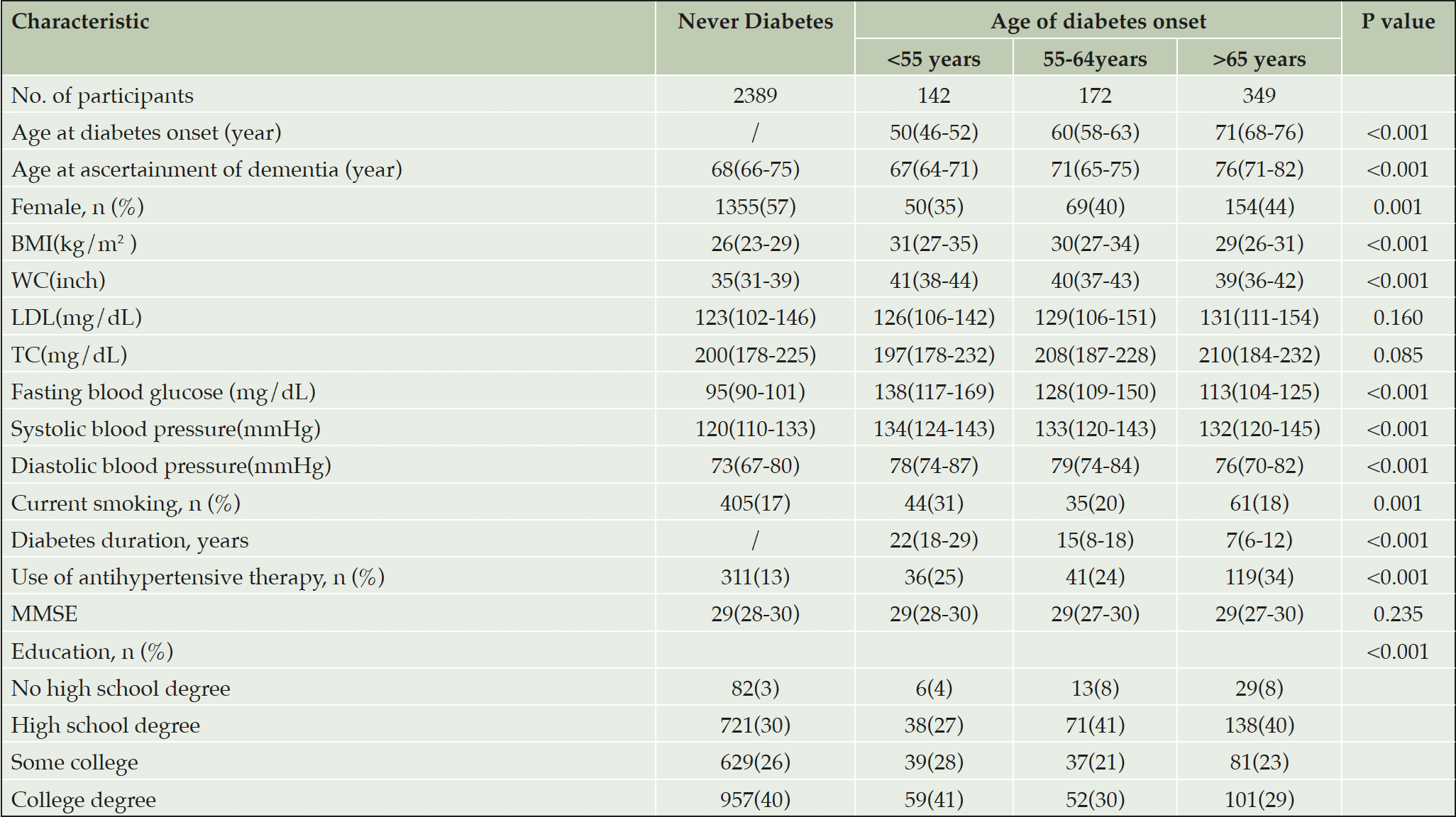
The characteristics of our study participants were shown in Table 1. Participants with early onset of diabetes had greater prevalence of hypertension and tended to smoke more. In addition, they were less likely to be women and more likely to have a high blood glucose, total cholesterol, SBP, WC and BMI. See Table 1.And the disease duration between different subgroup were shown in the supplement eTable 1.
Abbreviations: BMI, body mass index; WC, waist circumference. LDL, low-density lipoprotein, TC: total cholesterol, MMSE, mini-mental state examination; Values displayed are from the first examination cycle where these were available, except for the age at ascertainment of dementia and the age of diabetes onset
Early onset of Type 2 Diabetes and Risk for Dementia and Stroke
In the final research sample, 282 (9%) developed dementia, including 181 (6%) with AD dementia over a median follow-up of 12 years (interquartile range, 9 to 14 years), with an overall incidence rate of 8.30 per 1000 person-years. In addition, 206 (7%) cases of incident stroke were identified over a median follow-up of 12 years (interquartile range, 6 to 15 years) with an overall incidence rate of 6.06 per 1000 person-years.
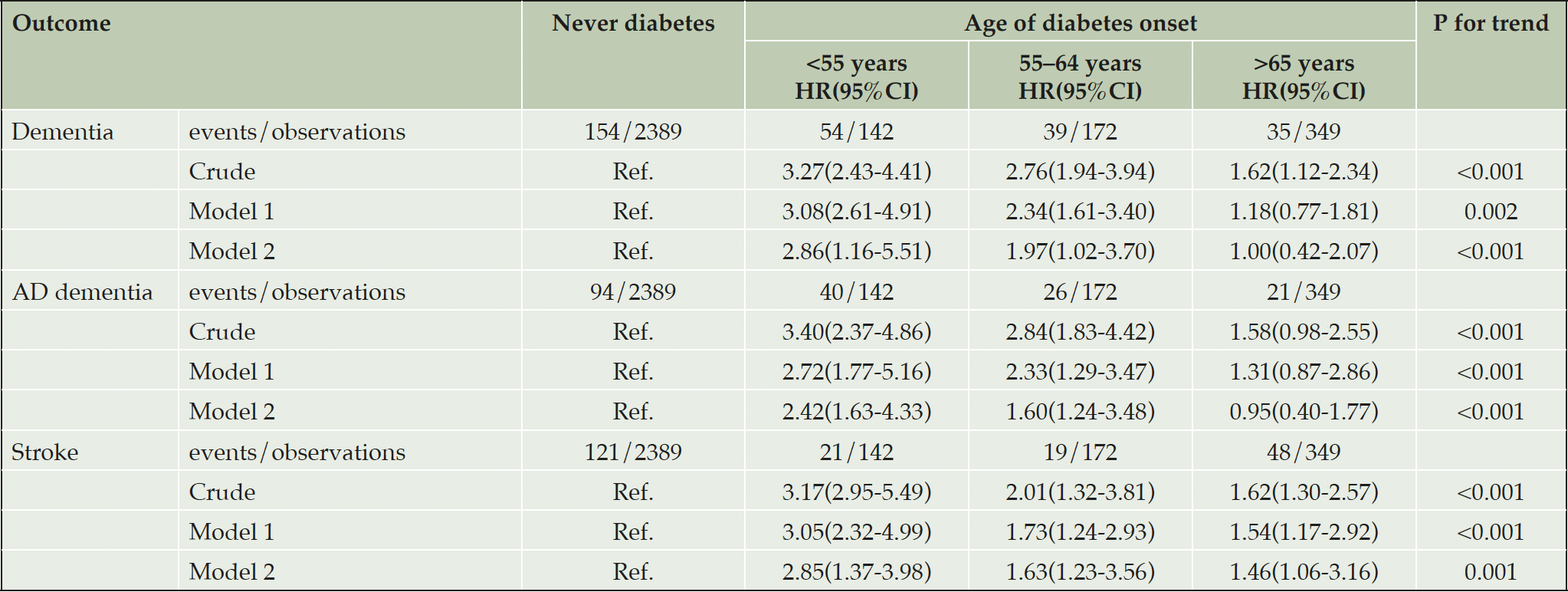
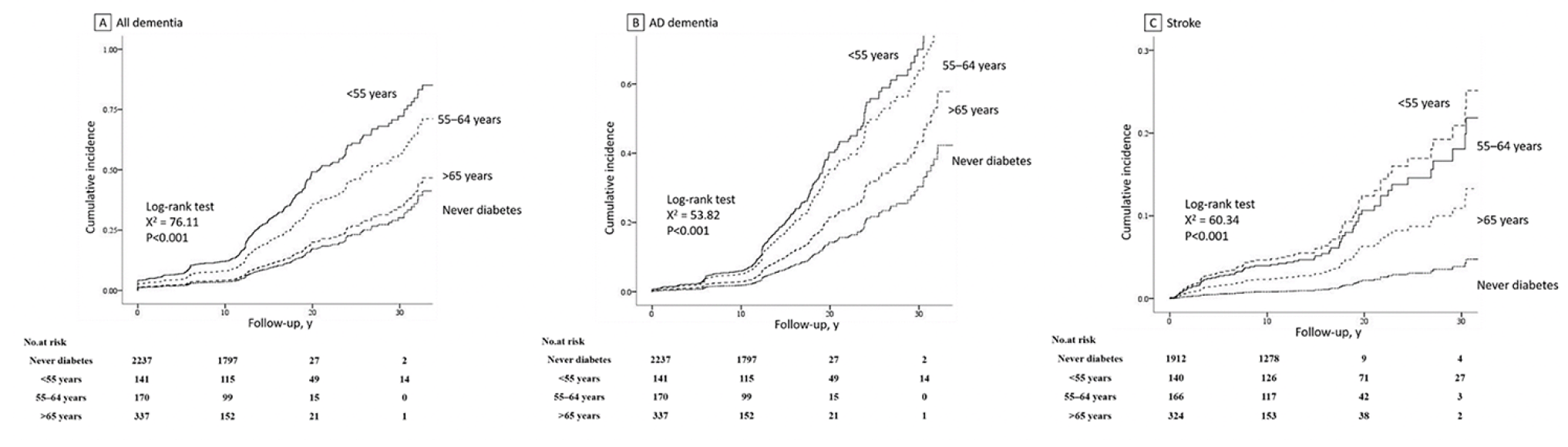
The trends of decreasing age of onset of diabetes in relation to increasing risk of dementia ,AD and stroke were statistically significant (P<0.05 for all, trend).When compared with individuals who never developed diabetes, people with onset of diabetes prior to age 55 years were associated with 2.86-fold higher risks (HR 2.86, 95%CI 1.16-5.51) of dementia and 2.42-fold higher (HR 2.42, 95%CI 1.63-4.33) of AD independent of potential confounders including sex, age at events, smoking status, SBP, BMI, total cholesterol, use of antihypertensive, education levels , MMSE and duration of diabetes. In the contrast, onset of diabetes at age over 65 years did not confer a statistically significant higher risk of dementia or AD (HR 1.00, 95%CI 0.47-2.10 for dementia, HR 0.96, 95%CI 0.41-1.78 for AD). And the results were similar when limiting to the risk of stroke, early onset of diabetes was associated with a greater risk of stroke than individual with late-onset diabetes (HR 2.85, 95%CI 1.37-3.98 with onset before age 55 years, HR 1.63, 95%CI 1.23-3.56 for 55-64 year, HR 1.46, 95%CI 1.06-3.16 for over 65 years), compared with no diabetes after multivariate adjustment (Table 2). Figure 2 showed the cumulative incidence curves for dementia, AD and stroke stratified by groups with different onset age of diabetes after full adjustment.
Model 1 Sex and age at events; Model 2 in addition for smoking status, SBP, BMI, TC, use of antihypertensive, education levels and duration of diabetes. All dementia and AD dementia were additionally adjusted for MMSE; Abbreviations: HR, hazard ratio; AD, Alzheimer disease; SBP: systolic blood pressure; BMI: body mass index; TC: total cholesterol; MMSE, mini-mental state examination; All the covariates were based on data from first examination at which measures and assessments were available.
Abbreviations: AD, Alzheimer disease. Data are for cumulative incidence of (A) all dementia, (B) Alzheimer disease dementia and (C) stroke among participants based on diabetes age of onset in the Framingham Heart Study. Adjustments were made for sex, age at events, smoking status, SBP, BMI, total cholesterol, use of antihypertensive, education levels and duration of diabetes.
Discussion
We conducted a longitudinal study of diabetes and dementia, AD and stroke risk in the FHS-OS and observed that a potentially important subset of diabetes may be defined based on the age of onset of diabetes. Specifically, it was found that when diabetes occurs prior to age 55 years as early-onset diabetes, there is a significantly greater life-time risk for dementia, AD and stroke compared with diabetes that manifests at a later age after adjustment for conventional risk factors.
Previous studies have confirmed that diabetes increases the risk of dementia, with HR values ranging from 1.5 to 2.0. To the end, a meta-analysis of 144 prospective studies showed a significant association between diabetes and increased risk of all-cause dementia (RR: 1.43, 95%CI: 1.33–1.53, I2 =79%) and AD (RR: 1.43, 95% CI: 1.25-1.62, I2 =81%) (7). However, it is well-known that diabetes is a heterogeneous disorder in terms of its natural history (21). Individuals with diabetes can vary widely in their disease course and complications-and this variation poses ongoing clinical challenges for diagnosing and managing affected persons (22). Thus, as part of efforts to identify higher-risk disease subgroups amid heterogeneity, only a few prior studies have assessed the effects of earlier versus later onset of diabetes on the dementia risk posed, the results have varied greatly and most were limited in the type 1 diabetes (T1D) in adolescents and the cognitive function instead of the clinical outcomes. On the one hand, a cross-sectional study recruiting 50 subjects with T1D with 30 healthy controls (ages between 7 and 16 years) suggested that subjects with early-onset T1D had significantly poorer performance than controls on most subtests of memory, intelligence and executive functioning as measured by the Benton Visual Retention Test (BVRT), Wechsler Intelligence Scale for Children (WISC), and Wisconsin Card Sorting Test (WCST) (23). A meta-analysis including 2,144 children consisted of 1,393 study subjects with type 1 diabetes and 751 control subjects from 19 studies demonstrated that cognitive effects are most pronounced and pervasive for children with early-onset diabetes with moderately lower performance across most cognitive domains compared with control subjects (24). On the other hand, although there was few study focusing on the relationship between early-onset diabetes and cognitive function. There were studies showed that early-onset diabetes suggested no burden of adverse outcomes risk factors (25). For instance, some studies have founded either a lower risk or no difference in risk for macrovascular complications among persons with earlier-versus later-onset diabetes after accounting for diabetes duration (26).
Given this situation, the early- versus late-onset diabetes were defined using objective clinical and biochemical data collected from serial examinations in a community-based cohort with over 30 years of prospective follow-up which allowed for a comprehensive assessment of long-term dementia risk in relation to age of diabetes onset. To our knowledge, our study is the first to demonstrate accelerated probability of developing all dementia, AD and stroke year-on-year at a population level in early-onset diabetes compared with late-onset based on much more accurate assessments for age of diabetes onset. And it is not a cross-sectional observations, but a long-term implications after full adjustment. Although diabetes onset in older age is of often associated with excess dementia or stroke risk factors that can further increase their risks and a longer time window to develop the events, diabetes onset at a younger age may represent a more aggressive subgroup that confers greater risks even after the disease duration and other confounders are accounted for.
There are several possible explanations for our main findings. Firstly, the metabolic disease followed the early-onset diabetes may harm cognitive function and lead to stroke. It is found in several studies that individuals with earlier- versus later-onset diabetes appear to have more pronounced clinical features of metabolic disease such as greater obesity, adiposity, dyslipidemia, hyperglycemia and hyperuricemia (27-29). And all of the metabolic disease are related to the development of dementia and stroke (30-33). Secondly, early-onset diabetes may be a phenotype distinct from late-onset diabetes with increased risk for dementia conferred by certain genetic variants besides APOE4 gene, rather than the same disease simply manifesting at a different point in life (34-37). Hence, the age of onset of diabetes can identify an especially high-risk subgroup of diabetes that confers a higher risk of dementia and stroke, although the mechanisms and certain genetic variants are incompletely understood.
The main strength of our study was the use of a community-based sample with detailed characteristics, accurate definition of dementia, AD and stroke and precise assessment for age of diabetes onset. Limitations of our study include the observational nature of the study and a small number of incident events. Second, we also did not account for the changes over time in the pharmacological approach to the primary and secondary prevention of dementia and stroke which may have affected the outcomes in our study. Thirdly, as our sample was of Caucasian decent, it is unclear how our results generalize to other ethnic groups. Fourthly, although, many sociodemographic variable were adjusted, there may be residual confounding by variables that cannot be measured with precision or were not available in the public dataset.
Conclusions
To sum up, the findings of this research demonstrate that relatively early-onset diabetes was associated with an increased risk of all dementia, AD dementia and stroke independent from multiple demographic factors. These findings have implications for prioritizing efforts to reduce dementia, AD and stroke risk in persons with prevalent diabetes-particularly younger individuals and the age of diabetes onset could represent a potentially indicator for prevention measures. It is upon future research to confirm our findings and to determine the targeted interventions and mechanism.
Data Availability
Data described in the manuscript, code book, and analytic code will not be made available because the authors are prohibited from distributing or transferring the data and codebooks on which their research was based to any other individual or entity under the terms of an approved NHLBI Framingham Heart Study Research Proposal and Data and Materials Distribution Agreement through which the authors obtained these data.
What this study adds to the literature
Relatively early-onset diabetes (age<55years) was associated with an increased risk of all dementia, AD dementia and stroke. Prioritizing efforts should be made in persons with prevalent diabetes-particularly younger individuals and the age of diabetes onset could represent a potentially indicator for prevention measures.
Conflicts of Interest: The authors declare that they have no conflicts of interest.
Acknowledgments: The authors thank the National Heart, Lung, and Blood Institute Framingham Heart Study, Framingham, MA, USA and Chongqing Medical University, Chongqing, China.
Ethical Standards: The study procedures followed were in accordance with the ethical standards of the Institutional Review Board and the Principles of the Declaration of Helsinki.
Funding: Chongqing Medical University Scholarship Fund for Development of Young Talents (No. XRJH201901).
References
1. Cao Q, Tan CC, Xu W, Hu H, Cao XP, Dong Q, Tan L, Yu JT. The Prevalence of Dementia: A Systematic Review and Meta-Analysis. J Alzheimers Dis 2020;73, 1157-1166.
2. Udeh-Momoh C, Price G, Ropacki MT, Ketter N, Andrews T, Arrighi HM, Brashear HR, Robb C, Bassil DT, Cohn M, Curry LK, Su B, Perera D, Giannakopoulou P, Car J, Ward HA, Perneczky R, Novak G, Middleton L. Prospective Evaluation of Cognitive Health and Related Factors in Elderly at Risk for Developing Alzheimer’s Dementia: A Longitudinal Cohort Study. J Prev Alzheimers Dis 2019;6, 256-266.
3. Shimada H, Makizako H, Tsutsumimoto K, Doi T, Lee S, Suzuki T. Cognitive Frailty and Incidence of Dementia in Older Persons. J Prev Alzheimers Dis 2018;5, 42-48.
4. Burns A, Iliffe S. Dementia. BMJ 2009;338, b75.
5. James BD, Bennett DA. Causes and Patterns of Dementia: An Update in the Era of Redefining Alzheimer’s Disease. Annu Rev Public Health 2019;40, 65-84.
6. Bo A, Pouwer F, Juul L, Nicolaisen SK, Maindal HT. Prevalence and correlates of diabetes distress, perceived stress and depressive symptoms among adults with early-onset Type 2 diabetes: cross-sectional survey results from the Danish DD2 study. Diabet Med 2020;37, 1679-1687.
7. Xue M, Xu W, Ou YN, Cao XP, Tan MS, Tan L, Yu JT. Diabetes mellitus and risks of cognitive impairment and dementia: A systematic review and meta-analysis of 144 prospective studies. Ageing Res Rev 2019;55, 100944.
8. Huo X, Gao L, Guo L, Xu W, Wang W, Zhi X, Li L, Ren Y, Qi X, Sun Z, Li W, Ji Q, Ran X, Su B, Hao C, Lu J, Guo X, Zhuo H, Zhang D, Pan C, Weng J, Hu D, Yang X, Ji L. Risk of non-fatal cardiovascular diseases in early-onset versus late-onset type 2 diabetes in China: a cross-sectional study. Lancet Diabetes Endocrinol 2016;4, 115-124.
9. Kong X, Xing X, Zhang X, Hong J, Yang W. Early-onset type 2 diabetes is associated with genetic variants of beta-cell function in the Chinese Han population. Diabetes Metab Res Rev 2020;36, e3214.
10. Huang JX, Liao YF, Li YM. Clinical Features and Microvascular Complications Risk Factors of Early-onset Type 2 Diabetes Mellitus. Curr Med Sci 2019;39, 754-758.
11. Wilmot E, Idris I. Early onset type 2 diabetes: risk factors, clinical impact and management. Ther Adv Chronic Dis 2014;5, 234-244.
12. Feinleib M, Kannel WB, Garrison RJ, McNamara PM, Castelli WP. The Framingham Offspring Study. Design and preliminary data. Prev Med 1975;4, 518-525.
13. An Investigation of Coronary Heart Disease in Families: The Framingham Offspring Study. Am J Epidemiol 2017;185, 1093-1102.
14. Abraham TM, Pencina KM, Pencina MJ, Fox CS. Trends in diabetes incidence: the Framingham Heart Study. Diabetes Care 2015;38, 482-487.
15. Cheng YJ, Kanaya AM, Araneta MRG, Saydah SH, Kahn HS, Gregg EW, Fujimoto WY, Imperatore G. Prevalence of Diabetes by Race and Ethnicity in the United States, 2011-2016. JAMA 2019;322, 2389-2398.
16. Genuth SM, Palmer JP, Nathan DM. Classification and Diagnosis of Diabetes In Diabetes in America, rd, Cowie CC, Casagrande SS, Menke A, Cissell MA, Eberhardt MS, Meigs JB, Gregg EW, Knowler WC, Barrett-Connor E, Becker DJ, Brancati FL, Boyko EJ, Herman WH, Howard BV, Narayan KMV, Rewers M, Fradkin JE, eds., Bethesda (MD), 2018.
17. Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, Malanda B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract 2018;138, 271-281.
18. American Psychatric Association.Arlington V. Diagnostic and Statistical Manual of Mental Disorders.4th ed, American Psychiatric Publishing, 2000.
19. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984;34, 939-944.
20. Satizabal CL, Samieri C, Davis-Plourde KL, Voetsch B, Aparicio HJ, Pase MP, Romero JR, Helmer C, Vasan RS, Kase CS, Debette S, Beiser AS, Seshadri S. APOE and the Association of Fatty Acids With the Risk of Stroke, Coronary Heart Disease, and Mortality. Stroke 2018;49, 2822-2829.
21. Pearson ER. Type 2 diabetes: a multifaceted disease. Diabetologia 2019;62, 1107-1112.
22. Harreiter J, Roden M. [Diabetes mellitus-Definition, classification, diagnosis, screening and prevention (Update 2019)]. Wien Klin Wochenschr 2019;131, 6-15.
23. Abo-El-Asrar M, Andrawes NG, Rabie MA, El-Gabry DA, Khalifa AG, El-Sherif M, Abdel Aziz K. Cognitive functions in children and adolescents with early-onset diabetes mellitus in Egypt. Appl Neuropsychol Child 2018;7, 21-30.
24. Gaudieri PA, Chen R, Greer TF, Holmes CS. Cognitive function in children with type 1 diabetes: a meta-analysis. Diabetes Care 2008;31, 1892-1897.
25. Zoungas S, Woodward M, Li Q, Cooper ME, Hamet P, Harrap S, Heller S, Marre M, Patel A, Poulter N, Williams B, Chalmers J, group AC. Impact of age, age at diagnosis and duration of diabetes on the risk of macrovascular and microvascular complications and death in type 2 diabetes. Diabetologia 2014;57, 2465-2474.
26. Chan JC, Lau ES, Luk AO, Cheung KK, Kong AP, Yu LW, Choi KC, Chow FC, Ozaki R, Brown N, Yang X, Bennett PH, Ma RC, So WY. Premature mortality and comorbidities in young-onset diabetes: a 7-year prospective analysis. Am J Med 2014;127, 616-624.
27. Aguilar-Salinas CA, Rojas R, Gomez-Perez FJ, Garcia E, Valles V, Rios-Torres JM, Franco A, Olaiz G, Sepulveda J, Rull JA. Prevalence and characteristics of early-onset type 2 diabetes in Mexico. Am J Med 2002;113, 569-574.
28. Zheng L, Chen X, Luo T, Ran X, Hu J, Cheng Q, Yang S, Wu J, Li Q, Wang Z (2020) Early-Onset Type 2 Diabetes as a Risk Factor for End-Stage Renal Disease in Patients With Diabetic Kidney Disease. Prev Chronic Dis 2020;17, E50.
29. Clissold RL, Hamilton AJ, Hattersley AT, Ellard S, Bingham C. HNF1B-associated renal and extra-renal disease-an expanding clinical spectrum. Nat Rev Nephrol 2015;11, 102-112.
30. Sun Y, Ma C, Sun H, Wang H, Peng W, Zhou Z, Wang H, Pi C, Shi Y, He X. Metabolism: A Novel Shared Link between Diabetes Mellitus and Alzheimer’s Disease. J Diabetes Res 2020, 4981814.
31. Appleton JP, Scutt P, Sprigg N, Bath PM. Hypercholesterolaemia and vascular dementia. Clin Sci (Lond) 2017;131, 1561-1578.
32. Singh-Manoux A, Dugravot A, Shipley M, Brunner EJ, Elbaz A, Sabia S, Kivimaki M. Obesity trajectories and risk of dementia: 28 years of follow-up in the Whitehall II Study. Alzheimers Dement 2018;14, 178-186.
33. Kuzma E, Lourida I, Moore SF, Levine DA, Ukoumunne OC, Llewellyn DJ. Stroke and dementia risk: A systematic review and meta-analysis. Alzheimers Dement 2018;14, 1416-1426.
34. Huopio H, Miettinen PJ, Ilonen J, Nykanen P, Veijola R, Keskinen P, Nanto-Salonen K, Vangipurapu J, Raivo J, Stancakova A, Mannisto J, Kuulasmaa T, Knip M, Otonkoski T, Laakso M. Clinical, Genetic, and Biochemical Characteristics of Early-Onset Diabetes in the Finnish Population. J Clin Endocrinol Metab 2016;101, 3018-3026.
35. Li M, Gong S, Han X, Zhang S, Ren Q, Cai X, Luo Y, Zhou L, Zhang R, Liu W, Zhu Y, Zhou X, Sun Y, Li Y, Ma Y, Ji L. Genetic variants of ABCC8 and phenotypic features in Chinese early onset diabetes. J Diabetes, 2020.
36. Olaiya MT, Wedekind LE, Hanson RL, Sinha M, Kobes S, Nelson RG, Baier LJ, Knowler WC. Birthweight and early-onset type 2 diabetes in American Indians: differential effects in adolescents and young adults and additive effects of genotype, BMI and maternal diabetes. Diabetologia 2019;62, 1628-1637.
37. Bansal V, Gassenhuber J, Phillips T, Oliveira G, Harbaugh R, Villarasa N, Topol EJ, Seufferlein T, Boehm BO. Spectrum of mutations in monogenic diabetes genes identified from high-throughput DNA sequencing of 6888 individuals. BMC Med 2017;15, 213.