H. Fillit1,2, S. Seleri Assunção3, T. Majda3, C.D. Ng3, T.M. To3, I.M. Abbass3, K. Raimundo3, C. Wallick3,4, O.V. Tcheremissine5
1. Alzheimer’s Drug Discovery Foundation, New York, NY, USA; 2. Departments of Geriatric Medicine and Palliative Care, Medicine, and Neuroscience, Icahn School of Medicine at Mount Sinai, New York, NY, USA; 3. Genentech, Inc., a member of the Roche Group, South San Francisco, CA, USA; 4. Neurocrine Biosciences, Inc., San Diego, CA, USA; 5. Department of Psychiatry, Atrium Health Behavioral Health Charlotte, Charlotte, NC, USA
Corresponding Author: Thomas Majda, PharmD, MS, 2104 Connor Way, Lake Saint Louis, MO 63367, P: 314-917-5791, E: majdat@gene.com
J Prev Alz Dis 2024;5(11):1251-1259
Published online June 21, 2024, http://dx.doi.org/10.14283/jpad.2024.115
Abstract
BACKGROUND: Linking data from clinical trials and real-world claims may improve the robustness of trial data and provide information on the health, economic, and societal impacts of a disease.
OBJECTIVE: To report on the feasibility of linking trial data to Medicare claims data in early symptomatic Alzheimer’s disease (AD) in the US.
DESIGN AND SETTING: Alzheimer’s Disease Linkage to Real-World Evidence (AD-LINE) was a noninterventional cohort study that included participants recruited from the GRADUATE program whose trial data were linked to their Medicare claims.
PARTICIPANTS: AD-LINE participants were 66 years and older with early symptomatic AD (ie, mild cognitive impairment [MCI] due to AD or mild AD dementia) and were enrolled in the GRADUATE program and a Medicare fee-for-service or Medicare Advantage plan.
MEASUREMENTS: The Centers for Medicare & Medicaid Services linked participants’ clinical trial identifiers to their Medicare beneficiary identifiers using a deterministic, exact matching process. Demographics and clinical characteristics of the AD-LINE cohort at baseline were collected. Outcomes measured in this study included healthcare resource utilization derived from Medicare claims data.
RESULTS: In total, 147 participants across 21 US sites were invited to participate and 111 provided informed consent. Of those, 61 patients had linkable data (ie, Medicare beneficiary identifier), Medicare Parts A/B enrollment, and no health maintenance organization (HMO) enrollment in the year before trial entry. Of the 61 participants whose data were analyzed in this study, 30 had MCI due to AD and 31 had mild AD dementia. Participants in the MCI due to AD group had more healthcare resource utilization on average in the baseline period than those in the mild AD dementia group (29.9 [SD, 20.9] vs 24.5 claims [SD, 12.3]). In an ad hoc analysis, a relatively high concordance (85.3%) was seen between the rates of clinically confirmed AD diagnosis and evidence of AD diagnosis in claims data.
CONCLUSION: This linkage process may serve as a proof of concept for researchers interested in linking clinical trial and real-world claims data. The lessons learned from AD-LINE and innovation of data linkage approaches may encourage key stakeholders to link data in the future.
Key words: Healthcare resource utilization, early Alzheimer’s disease, data linkage, clinical trial, real-world evidence.
Introduction
Randomized, controlled phase 3 trials are the gold standard to establish the efficacy and safety of medical interventions; however, they are logistically complex, expensive, and often lack generalizability to the real world (1, 2). Conversely, real-world data (RWD) derived from a broad range of sources including insurance claims, electronic medical records (EMR), registries, and patient surveys provide valuable information about the economic impact, healthcare resource utilization (HCRU), and patient-centric outcomes (eg, quality of life) associated with treatments (3, 4). The US Food and Drug Administration is increasingly receptive to accepting real-world evidence (RWE) in submission packages (5, 6). In fact, 25 of the 26 biologics license applications and new drug applications approved in the first 6 months of 2021 included RWE (5). Linking clinical trial data to real-world claims data may improve the relevance and comprehensiveness of evidence derived from clinical trials at a fraction of the cost, with minimal burden to sites and participants and without compromising study integrity or the collection of safety and efficacy data (7-9).
Research in the field of chronic disease demonstrates that linking clinical data to RWD can be an effective means of noninterventional observation, offering an opportunity to estimate the broader economic and societal impacts of a disease (10). Sponsors use clinical trial data and linked RWE to enhance evidence generation in a variety of ways. Clinical trial data and linked claims can be used to measure long-term outcomes after a trial has ended (10, 11); determine the cost-effectiveness of a new treatment (12); support payer health technology assessments; and help inform treatment coverage, access, and reimbursement decisions (13).
Clinical trial information can be enriched by linking clinical trial data and RWE, and tokenization in RWD collection is another approach used to increase the robustness of clinical trial information. In the context of clinical trials, tokenization is the process of deidentifying a participant’s personally identifiable information and generating an encrypted token. The token ID is typically an alphanumeric code based on personally identifiable information but does not contain any identifiable details. Once a token ID is assigned, deterministic and probabilistic matching can be used to link the same individual across multiple datasets (4, 14, 15). Tokenization allows researchers to link outcomes in clinical trials to RWD or medical history, and therefore creates a more complete overview of the person’s health history (4, 14, 16 ,17). Linkage to broader RWD sources would require substantial resources (eg, additional costs and partnerships with third-party tokenization vendors) and innovative data tokenization methods. Additionally, linkages to other sources of RWD, such as wearables, EMR, and social media data, may provide valuable insights (4).
Long-term follow-up and linkage to registries and other sources of regularly collected administrative data have been used most often in oncology and cardiovascular diseases; however, linkages to claims or EMR data are less common (18). To our knowledge, no study has linked phase 3 clinical trial data with real-world claims data in participants with early symptomatic AD treated with a disease-modifying therapy (DMT). Given that AD is a growing public health issue with an evolving treatment landscape, more linkage studies are needed for patients with early symptomatic AD as they receive new treatments. It is estimated that up to 6.9 million people in the US 65 years and older are currently living with AD or related dementia, with an additional 13.9 million people living with MCI due to any cause (19); epidemiologic studies show that the majority of dementia and MCI cases in individuals 65 or older are due to AD (20, 21). By 2050, about 12.7 million Americans over 65 will be living with AD dementia and 19.3 million will be living with MCI (19). The prevalence of MCI is only expected to grow; therefore, research has been conducted to understand the humanistic and economic burden of early symptomatic AD (22). An analysis of healthcare use and expenditures covered by Medicare demonstrated increased costs and resource use (ie, increased number and duration of hospitalizations, and increased physician visits and home health services) in those diagnosed with MCI compared with propensity-matched controls (23). Further, those with MCI may experience a lower quality of life and may experience greater depression and stress (24). Given the rising prevalence of MCI and recent approval of DMTs, and with many ongoing trials evaluating new treatments in early symptomatic AD, a broadened understanding of the economic and societal impact of new medications is needed now more than ever. By delaying AD dementia onset, DMTs have the potential to reduce the humanistic and economic burden of MCI; however, estimates regarding the potential extent of the impact are based on simulation models (25, 26). Linking early symptomatic AD clinical trial data with RWD may provide critical information about the real-world impact of DMTs as they continue to enter the market.
We designed the Alzheimer’s Disease Linkage to Real-World Evidence (AD-LINE) study, a first-of-its-kind direct linkage study between Medicare claims data and phase 3 clinical trial data from the GRADUATE program. The aim of this publication is to report on the feasibility of linking trial data to Medicare claims data in early symptomatic AD in the US. The methodology of and lessons learned from the AD-LINE study offer guidance on a novel process of linking clinical trial data to Medicare claims data.
Methods
GRADUATE Program Description
The GRADUATE program was developed to assess the safety and efficacy of gantenerumab in individuals with early symptomatic AD (27). Gantenerumab is a fully human anti-amyloid-beta (Aβ) IgG1 monoclonal antibody for subcutaneous administration with highest affinity for aggregated Aβ, including oligomers, fibrils, and plaques, and was designed to promote clearance of amyloid plaques in the brain (28).
For the purposes of this manuscript, the GRADUATE program includes 3 studies:
GRADUATE I and II (NCT03444870 and NCT03443973), and POST-GRADUATE (NCT04374253). GRADUATE I and II were 2 parallel, identical, phase 3, randomized, placebo-controlled studies designed to assess the safety and efficacy of gantenerumab in participants with early symptomatic AD confirmed by the presence of Aβ pathology via amyloid positron emission tomography or cerebrospinal fluid assessment (27). Additional key inclusion criteria for the GRADUATE studies included a Mini-Mental State Examination score greater than or equal to 22 and Clinical Dementia Rating-Global Score of 0.5 or 1.0 (27). The primary endpoint of these studies was mean change from baseline to Week 116 in the Clinical Dementia Rating-Sum of Boxes (CDR-SB) (27). Patients who completed one of the double-blind studies had the option to enter into POST-GRADUATE, an open-label extension trial, for an additional 24 months (28). The GRADUATE I and II studies enrolled 1965 patients and concluded in 2022. The primary endpoint was not met, and the GRADUATE program was subsequently terminated (27).
AD-LINE Study Description
AD-LINE was a noninterventional cohort study designed to link data from individuals who were enrolled in the GRADUATE program—more specifically, GRADUATE I and GRADUATE II—to RWD from Medicare claims. The Centers for Medicare & Medicaid Services (CMS) Research Data Assistance Center (ResDAC) was contacted early in the process for advice on the study protocol and insight into CMS requirements. ResDAC provides complete claims data for Medicare fee-for-service and a subset of data from Medicare Advantage (MA) plans. Institutional Review Board approval of the study protocol and informed consent form were initiated before sites were approached to engender trust in data privacy and to promote site participation. Only one visit was required for the AD-LINE study, in which the participants provided informed consent and unique identifiers of protected health information (PHI). Participants continued to attend regular follow-up visits per the GRADUATE program studies. Data collection from the clinical trials and/or claims in eligible participants (described below) were planned as follows:
1. Claims data from one year prior to GRADUATE randomization;
2. Clinical data from the GRADUATE I and II studies and claims data (up to 24 months);
3. Clinical data from the POST-GRADUATE open-label extension and claims data (up to 24 months); and finally,
4. Claims data for 10 years for each patient or until 90% of participants discontinue AD-LINE due to study withdrawal or death.
The study was executed in 2 stages; in the first, we assessed the feasibility of linking the 2 datasets in 32 patients. Once we demonstrated that these datasets could be linked, we reopened enrollment. Data from patients in stage 1 were also included in stage 2. The original primary objective of the AD-LINE study was to evaluate the association between cognitive and/or functional declines and HCRU and/or associated medical costs using participants’ GRADUATE CDR-SB assessments and their Medicare claims data. Secondary objectives were to evaluate whether differences existed in HCRU and cost over a prespecified period between patients with varying functional and/or cognitive decline rates, to describe the differences in HCRU and associated costs over time, and to assess the association between rates of cognitive and functional decline over time to clinically meaningful outcomes such as time to skilled nursing facility admission. Ad hoc analyses of baseline HCRU and concordance of AD-related diagnoses in trial data vs in claims data among the AD-LINE cohort were also conducted.
Study Participants
The AD-LINE cohort comprised GRADUATE program patients 66 years or older who were diagnosed with early symptomatic AD (ie, MCI due to AD or mild AD dementia) and were enrolled in Medicare fee-for-service plans or select MA plans. Participants were required to provide informed consent allowing release of PHI, such as a combination of their Medicare insurance number (ie, Medicare beneficiary identifier [MBI]), date of birth, and sex, and Medicare claims data to a Health Insurance Portability and Accountability Act (HIPAA)-certified contract research organization.
Data Linkage Process
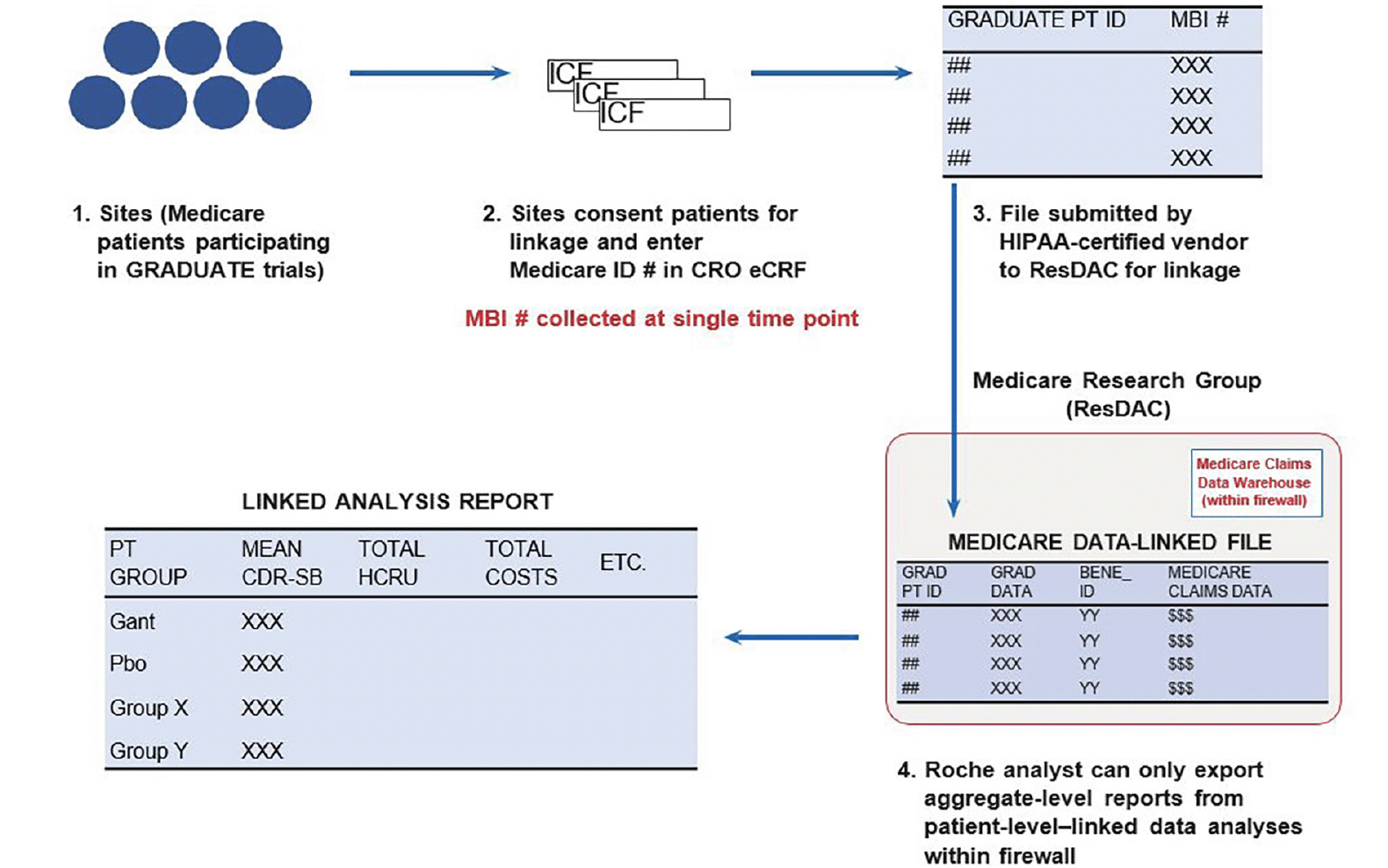
Participating sites entered the AD-LINE participants’ PHI into a secure electronic case report form. A HIPAA-certified contract research organization collected the PHI through a dedicated AD-LINE study database (separate from the GRADUATE trial databases), stored it on a secure server, and sent the files containing the participants’ clinical trial subject identifier and MBI to the CMS ResDAC for linkage. CMS linked the clinical trial identifier to the MBI using a deterministic, exact matching process (Figure 1). Detailed information about AD progression (ie, clinical outcomes assessments measuring cognitive and functional decline) was collected from the clinical trial assessments, while a single data analyst analyzed aggregate HCRU behind a firewall, using Medicare claims data. The data were reviewed by ResDAC before being released to ensure adequate deidentification. Per CMS reporting restrictions, to protect the confidentiality of beneficiaries, no cell containing a value of 1 to 10 can be reported directly; therefore, cells with derivable non-zero values <11 were suppressed (29).
BENE_ID, beneficiary identifier; CDR-SB, Clinical Dementia Rating-Sum of Boxes; CRO, contract research organization; eCRF, electronic case report form; HCRU, healthcare resource utilization; HIPAA, Health Insurance Portability and Accountability Act; ICF, informed consent form; ID, identifier; Gant, gantenerumab; GRAD, GRADUATE; MBI, Medicare beneficiary identifier; Pbo, placebo; PT, patient; ResDAC, Research Data Assistance Center.
Results
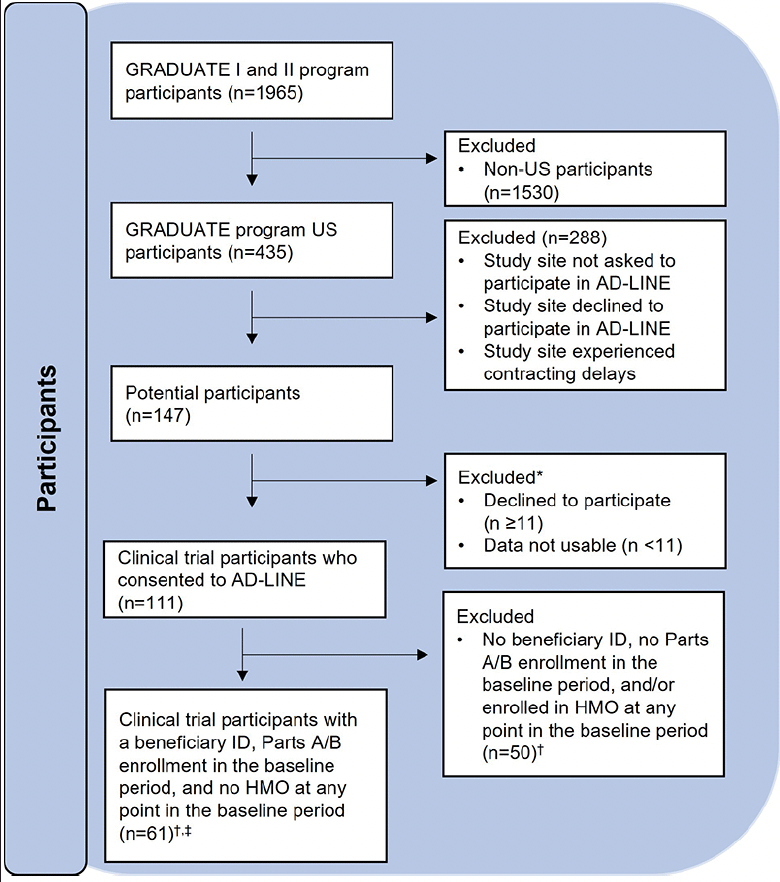
Thirty sites in the US were asked to participate in the AD-LINE study. Twenty-two agreed; however, one experienced contracting delays and therefore, participants were recruited from 21 US sites. In total, 147 participants were invited to participate in the AD-LINE study and 111 provided consent for their data to be linked to claims for this study. Claims data from 61 of the 111 participants were analyzed after ensuring linkable MBIs, Medicare Parts A/B enrollment, and no health maintenance organization (HMO) enrollment in the year prior to trial entry (Figure 2). Participants with HMO enrollment were excluded from analysis, as complete HCRU for individuals enrolled in HMOs may not be captured due to CMS reporting requirements (30).
*Suppressed to comply with CMS ResDAC cell size repression policy. †Baseline period is defined as a year prior to clinical trial entry to the day before clinical trial entry. ‡<11 participants are designated as Medicare Advantage enrollees by the finder file. CMS, Centers for Medicare & Medicaid Services; HMO, health maintenance organization; ID, identifier; ResDAC, Research Data Assistance Center.
Demographics and Clinical Characteristics
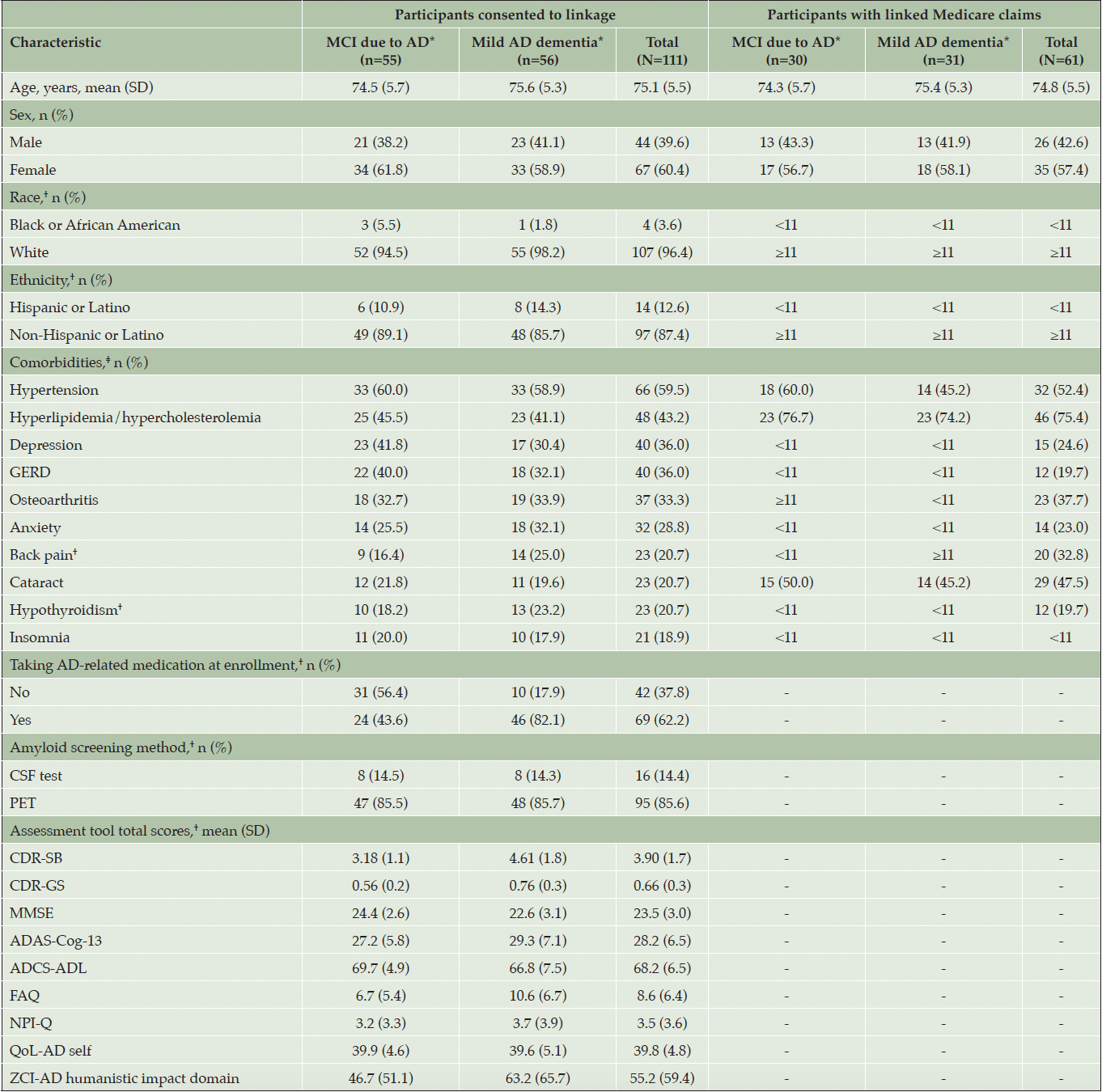
Baseline demographic and clinical characteristics for the 111 consented participants are listed in Table 1. In this patient population at baseline, the mean (SD) age was 75.1 (5.5) years, 67 (60.4%) patients were female, and the majority were White (96.4%) and non-Hispanic or Latino (87.4%). Fifty-five participants had MCI due to AD (ie, the MCI due to AD cohort) and 56 had mild AD dementia (ie, the mild AD dementia cohort), as per the investigators’ criteria. Participants in the mild AD dementia cohort were more likely to be taking medications approved for AD dementia than those in the MCI due to AD cohort. Other demographic and clinical characteristics were similar between the MCI due to AD and mild AD dementia groups.
*Values determined by trial investigators. †Some values in this table have been suppressed to comply with CMS ResDAC cell size repression policy. ‡Comorbidities were assessed in any type of claim and in any position within a claim. Top 10 comorbidities occurring in total consented trial participants. Abbreviations: AD, Alzheimer’s disease; ADAS-Cog-13, Alzheimer’s Disease Assessment Scale – Cognitive Subscale; ADCS–ADL, Alzheimer’s Disease Cooperative Study–Activities of Daily Living; CDR-GS, Clinical Dementia Rating-Global Score; CDR-SB, Clinical Dementia Rating-Sum of Boxes; CMS, Centers for Medicare & Medicaid Services; CSF, cerebrospinal fluid; FAQ, Functional Activities Questionnaire; GERD, gastroesophageal reflux disease; MCI, mild cognitive impairment; MMSE, Mini-Mental State Examination; NPI-Q, Neuropsychiatric Inventory Questionnaire; PET, positron emission tomography; QoL-AD, Quality of Life-Alzheimer’s Disease; ResDAC, Research Data Assistance Center; SD, standard deviation; ZCI-AD, Zarit Caregiver Interview for Alzheimer’s Disease.
On average, the AD-LINE cohort was slightly older than the global GRADUATE I and II trial participants (mean age 75.1 years vs 71.7 years), which can be attributed to the AD-LINE inclusion criterion for age at enrollment (66 years or older) versus that of the GRADUATE studies (50 years or older) (27). In addition, the AD-LINE cohort was less racially and ethnically diverse than the global GRADUATE trial population. As expected, the demographic characteristics of this AD-LINE cohort were more similar to the US GRADUATE participants compared with the global GRADUATE participants. Among all US GRADUATE participants, the mean age was 73.0 years, and the majority were White (96.3%) and not Hispanic or Latino (89.7%) (31). Differences in comorbidities were noted between the 111 consenting trial participants and 61 linked participants. Specifically, the most common comorbidities among the 111 consenting trial participants were hypertension, depression, gastroesophageal reflux disease, osteoarthritis, and anxiety, whereas hyperlipidemia/hypercholesterolemia, hypertension, cataract, osteoarthritis, back pain, and depression were the most common in the claims of the 61 linked participants (Table 1).
HCRU Data at Baseline in the Linked Cohort
Of the 61 participants who had linkable MBIs, Medicare Parts A/B enrollment, and no HMO enrollment in the year prior to trial entry, 30 had MCI due to AD and 31 had mild AD dementia. The mean (SD) age of this cohort was 74.8 (5.5) years and 35 (57.4%) patients were female. No major demographic differences were seen between the participants in these 2 stages of early symptomatic AD (as determined by GRADUATE trial investigators).
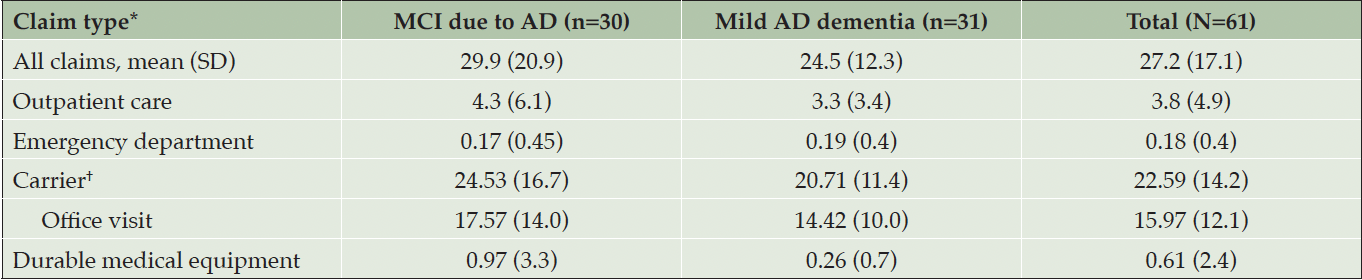
Participants in the MCI due to AD cohort had an average of 29.9 claims in the year before trial entry (ie, the baseline period) compared with 24.5 claims for participants in the mild AD dementia cohort (Table 2). No claims for inpatient care, home health agency, skilled nursing facility, or hospice were identified in either cohort. A sensitivity analysis of carrier claims was done in an attempt to investigate why the MCI due to AD group had higher HCRU on average than the mild AD dementia group. There were 1378 carrier claims in the baseline period across the 61 patients; of those, 974 were office visit claims. AD was the primary diagnosis in the mild AD group for 6.1% of carrier claims and 6.9% of office visit claims compared with 2.6% and 2.7%, respectively in the MCI due to AD group (Supplemental Table 1). Carrier claims include fee-for-service claims submitted by professional providers (ie, physicians, physician assistants, clinical social workers, nurse practitioners). The distribution of carrier claims in the baseline period was investigated as total number of claims in order to gain insight into claim categories that would otherwise not be reportable if investigated as average per patient due to CMS reporting restrictions.
*During this period, there were no claims in either group for inpatient care, home health agency, skilled nursing facility, or hospice care. †Carrier file includes fee-for-service claims submitted by professional providers, including physicians, physician assistants, clinical social workers, nurse practitioners. Abbreviations: AD, Alzheimer’s disease; MCI, mild cognitive impairment; SD, standard deviation.
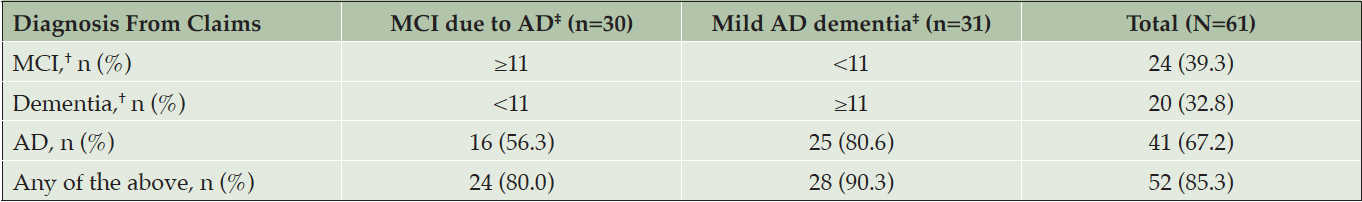
Claims containing an MCI, dementia, or AD diagnosis code in the baseline period (Table 3) were collected to compare rates of real-world diagnoses to diagnoses as determined by GRADUATE trial investigators. Collectively, 85.3% of the overall participants had a claim with an MCI, dementia, or AD diagnosis within the 12 months prior to clinical trial entry, whereas 80.6% of participants entering the trial with mild AD dementia had a claim with an AD diagnosis. For all participants, 39.3% had a claim for MCI, 32.8% had a claim for dementia, and 67.2% had a claim for AD.
*Diagnosis codes were assessed in any position within a claim. †Suppressed to comply with CMS ResDAC cell size repression policy. ‡Values determined by trial investigators. Abbreviations: AD, Alzheimer’s disease; CMS, Centers for Medicare & Medicaid Services; MCI, mild cognitive impairment; ResDAC, Research Data Assistance Center.
Descriptive analyses were planned to address the primary objective of assessing the association between the CDR-SB from the GRADUATE clinical trial data and health economic data using the linked Medicare claims. For the intended comparative analyses, statistical adjustment for potential confounders was planned using generalized regression models (ie, Poisson models for rates and Cox or other survival models for time-to-event analyses), with the intent to use reweighting to address informative loss to follow-up or selection biases in as-treated analyses. However, the GRADUATE I and II trials did not meet their primary endpoint and the sample size of the AD-LINE cohort is small, which limits the data that can be reported per the CMS ResDAC cell size repression policy. Therefore, many analyses planned in the protocol will not be conducted. Additionally, the AD-LINE cohort will likely only decrease over time, with increasing enrollment in MA plans and, by extension, increasing enrollment in HMOs (32).
Discussion
Integrating clinical trial data with real-world administrative claims data makes it feasible to establish a specialized database that combines clinical outcomes with extensive HCRU data over a prolonged period. This connection significantly enhances research capabilities and enables the early assessment of RWE regarding treatment outcomes.
AD-LINE explored the feasibility of linking phase 3 clinical trial data from a potential registrational program with Medicare claims data in participants with early symptomatic AD. Data from 61 of 111 (55.0%) consenting participants were used in this analysis to evaluate HCRU using Medicare claims data collected for the year preceding GRADUATE trial entry. No major demographic differences existed between the MCI due to AD and mild AD dementia groups. As expected, the rate of participants in the mild AD dementia cohort taking medications approved for AD dementia was higher versus participants in the MCI due to AD cohort, given that these medications are not approved in patients with MCI. A relatively high concordance was observed between the rates of clinically confirmed MCI due to AD or mild AD dementia diagnosis and evidence of MCI, dementia, or AD diagnosis in real-world US claims data, supporting the use of RWD in early symptomatic AD. One explanation of these high concordance rates could be that a number of participants who had been diagnosed with MCI, dementia, or AD in the real world then went on to enroll in the GRADUATE trials. However, it is important to note that the low rate of MCI diagnosis codes in claims for both the MCI due to AD and mild AD dementia groups highlights the need for earlier identification and documentation of cognitive decline. An observational analysis of Medicare claims from 2015 to 2019 demonstrated a low detection rate of early AD in Medicare populations. In fact, approximately 7.4 million of 8 million Medicare beneficiaries may have undiagnosed MCI (33).
Data from large cross-sectional and observational studies have indicated that HCRU increases over time as AD progresses (34, 35). In contrast with this published data, participants in the MCI due to AD group had more claims on average in the baseline period than the mild AD dementia group. We hypothesize that this could be explained in part due to the small sample size. Another potential explanation for this finding may be that patients who are experiencing symptoms of MCI may undergo more diagnostic procedures and/or specialist referrals before the etiology of their MCI symptoms can be determined. It is also possible that the population recruited to the GRADUATE program was at the stage right after formal AD diagnosis, when there is a marked increase in HCRU based on providers’ desire to compile a differential diagnosis for cognitive impairment disorders (36, 37). Evidence from real-world studies indicates that Medicare beneficiaries experience an increase in hospitalizations in the months and years leading up to a formal diagnosis of AD or MCI (23, 37, 38). This trend also contrasts with our findings, in which no inpatient claims were filed during the baseline period. The lack of claims during this period may be because the AD-LINE cohort is a select group of patients originally established based on clinical trial inclusion and exclusion criteria; therefore, the cohort is relatively healthy when compared with community-dwelling individuals with early symptomatic AD. The increased distribution in the total number of carrier and office visit claims in the baseline period was as expected for this population.
This study had several limitations. Although GRADUATE I and II were large phase 3 studies, the recruitment sites included several specialized AD centers, and the numbers of both recruitment sites and participants were limited. The AD-LINE study protocol was not integrated within the original GRADUATE I and II protocols, which increased logistical complexity. The cost and resources associated with enrolling each new site and participant were high; therefore, we strategically selected a group of sites and conveyed the importance of this work and the limited potential of data compromise. Although 30 sites were initially invited to participate, 8 later declined for reasons including concerns around volume of ongoing clinical trial work, uncertainty about patients agreeing to share claims data, or lack of interest in the study. Several sites did not want to ask patients for their insurance information, including Medicare insurance information. Some study sites needed clarification on which insurance number was needed for linkage; therefore, on-site training about the identification of Medicare numbers was valuable in site recruitment. Additionally, on-site training addressing how to communicate the importance of this work to site personnel and prospective participants and offering detailed information on the steps taken to mitigate data risks were helpful.
AD-LINE endpoints were not included in the protocols of the GRADUATE studies, which created complexities and incurred additional expenses. Our analysis was limited to a small sample of a specific group of patients in a controlled clinical trial setting. HCRU in clinical trials may vary significantly from HCRU in the real world, and our results may not be generalizable due to several factors (eg, subpopulation from a clinical trial and a small sample size). Furthermore, the AD-LINE cohort is not representative of US population demographics for MCI and AD regarding race and ethnicity (19). Although inclusive research initiatives were enacted to recruit diverse patient populations in the GRADUATE trials, study sites encountered barriers such as a lack of uniform outreach measurement standards. The design of the ALUMNI-AD trial, a study to evaluate the safety and efficacy of subcutaneous gantenerumab in underrepresented populations with early symptomatic AD, incorporated many solutions to collaborate with community partners and reduce these barriers; however, because the GRADUATE trials did not meet their primary endpoints, the study was not conducted (39). The barriers and facilitators of inclusive research identified through the GRADUATE and ALUMNI-AD initiatives will be used to improve the ethnic and racial diversity of future clinical program populations (31).
Lessons learned from the AD-LINE study can be implemented in future linkage trials. For example, we allowed MA patients, including those enrolled in HMOs, to enter the AD-LINE study. However, at the time of data analysis, we could not be confident that we were adequately capturing the full picture of HCRU for these patients. This is aligned with previous studies of HCRU using CMS claims (40, 41). Although it may limit generalizability, in the future, we would exclude participants enrolled in MA plans from recruitment in any type of linkage effort to assess HCRU using Medicare data. To ensure an adequate sample size, it may be beneficial to set a minimum sample size to account for MA patients and attrition over time including patients who will move from fee-for-service to MA. Furthermore, enrollment in the AD-LINE study occurred at a different time point from the GRADUATE trials, which may have limited recruitment. Recruitment in future linkage studies may be more robust if the collection of HCRU through claims data were an optional exploratory endpoint of the main clinical trial protocol. Prospectively designing trials that utilize passively collected data from sources like claims or registries present a unique opportunity to combine the advantages of both data types. While including the AD-LINE study in the main clinical trial protocol may have increased sample size and limited logistical complexities, a majority of the sites invited agreed to participate and stated they did not perceive the AD-LINE study as burdensome, and there was a very high patient consent rate.
The AD-LINE study was initiated in 2019, when linking clinical trial data to RWD was still in early conceptual phases. Since 2019, others have conducted linkage studies of clinical trial data and RWD in a variety of disease states including aortic stenosis (12), cardiovascular disease (11), cancer (7), and rheumatoid arthritis (10). Although this linkage study did not produce the expected results due to challenges related to small sample size and termination of the GRADUATE program, the valuable lessons learned and innovative nature of new data linkage approaches may encourage key stakeholders to seek additional opportunities to link data in the future. Novel approaches such as those outlined in the AD-LINE study have the potential to iteratively improve the ways in which clinical trials are conducted, and the AD-LINE study may serve as a proof of concept for clinical trial and RWD linkage in future trials.
The AD-LINE study used a novel, privacy-compliant, scalable approach for linking clinical trial data with Medicare claims to study the long-term economic impact of early AD progression in an attempt to better understand real-world impacts outside of the traditional clinical trial setting. The AD-LINE framework can be used to further investigate the use of tokenization in clinical trials to exponentiate the collection of real-world insights by connecting multidimensional data for each individual participant in a highly effective manner. The AD-LINE study may serve as a model for other researchers, providing valuable guidance on linking trial data with HCRU data—a connection that may offer critical information for healthcare professionals, payers, population health decision makers, and researchers. We hope that this work encourages others to pursue this claims data linkage process in phase 3 trials and that future studies with larger sample sizes will validate this approach.
Funding: The research described in this article was funded by Genentech, Inc. and F. Hoffmann-La Roche.
Acknowledgments: The authors wish to thank the patients who consented to participate in the study as well as the clinical trial sites for their collaboration. We would also like to thank Er Chen, David Evans, Deepa Lalla, Grace Leung, Peter Neumann, Rikisha Parekh, Vanessa Reddy, Jennifer Whiteley, and Rongrong Wang, who were involved in the early phase of the study. Medical writing and editorial support were provided by Andrea Morrello, Beth Miller, Clare Sonntag, and Jennifer Reed of Health & Wellness Partners, LLC, in accordance with the Good Publication Practice guidelines.
Disclosures: SSA, TM, CDN, TMT, IMA, and KR are employees of Genentech, Inc. and shareholders of F. Hoffmann-La Roche. CW was an employee of Genentech, Inc. and a shareholder of F. Hoffmann-La Roche at the time this study was conducted. HF is an unpaid consultant for Roche/Genentech. OVT is a consultant for Roche/Genentech and received research support from Athira, Eli Lilly, Concept, and Genentech/Roche.
References
1. Gabel M, Bollinger RM, Coble DW, et al. Retaining participants in longitudinal studies of Alzheimer’s disease. J Alzheimers Dis. 2022;87(2):945-955. doi: 10.3233/JAD-215710
2. Langbaum JB, Zissimopoulos J, Au R, et al. Recommendations to address key recruitment challenges of Alzheimer’s disease clinical trials. Alzheimers Dement. 2023;19(2):696-707. doi: 10.1002/alz.12737
3. Mitchell JM, Patterson JA. The inclusion of economic endpoints as outcomes in clinical trials reported to ClinicalTrials.gov. J Manag Care Spec Pharm. 2020;26(4):386-393. doi: 10.18553/jmcp.2020.26.4.386
4. Dagenais S, Russo L, Madsen A, Webster J, Becnel L. Use of real-world evidence to drive drug development strategy and inform clinical trial design. Clin Pharmacol Ther. 2022 Jan;111(1):77-89. doi: 10.1002/cpt.2480
5. Purpura CA, Garry EM, Honig N, Case A, Rassen JA. The role of real-world evidence in FDA-approved new drug and biologics license applications. Clin Pharmacol Ther. 2022;111(1):135-144. doi: 10.1002/cpt.2474
6. US Food and Drug Administration. Considerations for the use of real-world data and real-world evidence to support regulatory decision-making for drug and biological products: guidance for industry. Published August 2023. Accessed 22 May 2024. https://www.fda.gov/media/171667/download
7. Hay AE, Pater JL, Corn E, et al. Pilot study of the ability to probabilistically link clinical trial patients to administrative data and determine long-term outcomes. Clin Trials. 2019;16(1):14-17. doi: 10.1177/1740774518815653
8. Fillit H, Cummings J, Neumann P, McLaughlin T, Salavtore P, Leibman C. Novel approaches to incorporating pharmacoeconomic studies into phase III clinical trials for Alzheimer’s disease. J Nutr Health Aging. 2010;14(8):640-647. doi: 10.1007/s12603-010-0310-8
9. Chen Y, Tysinger B, Crimmins E, Zissimopoulos JM. Analysis of dementia in the US population using Medicare claims: Insights from linked survey and administrative claims data. Alzheimers Dement (N Y). 2019;5:197-207. doi: 10.1016/j.trci.2019.04.003
10. McGuiness CB, Boytsov NN, Zhang X, Wang X, Kannowski CL, Wade RL. Probabilistic linkage of randomized controlled trial data to administrative claims: a case study of patients from baricitinib clinical trials. Rheumatol Ther. 2021;8(2):793-802. doi: 10.1007/s40744-021-00302-2
11. Brennan JM, Wruck L, Pencina MJ, et al. Claims-based cardiovascular outcome identification for clinical research: results from 7 large randomized cardiovascular clinical trials. Am Heart J. 2019;218:110-122. doi: 10.1016/j.ahj.2019.09.002
12. Baron SJ, Wang K, House JA, et al. Cost-effectiveness of transcatheter versus surgical aortic valve replacement in patients with severe aortic stenosis at intermediate risk. Circulation. 2019;139(7):877-888. doi: 10.1161/CIRCULATIONAHA.118.035236
13. Roberts MH, Ferguson GT. Real-world evidence: bridging gaps in evidence to guide payer decisions. Pharmacoecon Open. 2021;5(1):3-11. doi: 10.1007/s41669-020-00221-y
14. Eckrote MJ, Nielson C, Lu M, et al. Linking clinical trial participants to their U.S. real-world data through tokenization: a practical guide. 26 January 2024. https://papers.ssrn.com/sol3/papers.cfm?abstract_id=4698358. Accessed 25 March 2024
15. Leshin J, Sanghvi A, Ravuri K, Owen M, Kho A. The impact of name transformation on match rates within a large consumer database. AMIA Annu Symp Proc. 2023;2022:692-699.
16. RWE, RWD, and tokenization for asset development: what biopharma teams need to know. https://www.syneoshealth.com/insights-hub/rwe-rwd-and-tokenization-asset-development-what-biopharma-teams-need-know. Accessed 8 January 2024
17. Weng I. Linking RWE to clinical trials. https://www.komodohealth.com/insights/linking-rwe-to-clinical-trials. Accessed 8 January 2024
18. Fitzpatrick T, Perrier L, Shakik S, et al. Assessment of long-term follow-up of randomized trial participants by linkage to routinely collected data: a scoping review and analysis. JAMA Netw Open. 2018;1(8):e186019. doi: 10.1001/jamanetworkopen.2018.6019
19. Rajan KB, Weuve J, Barnes LL, McAninch EA, Wilson RS, Evans DA. Population estimate of people with clinical ad and mild cognitive impairment in the United States (2020–2060). Alzheimers Dement. 2021;17(12):1966-1975. doi: 10.1002/alz.12362
20. Gillis C, Montenigro P, Nejati M, Maserejian N. Estimating prevalence of early Alzheimer’s disease in the United States, accounting for racial and ethnic diversity. Alzheimers Dement. 2023;19(5):1841-1848. doi: 10.1002/alz.12822
21. Alzheimer’s Association. 2023 Alzheimer’s disease facts and figures. Alzheimers Dement. 2023;19(4). doi: 10.1002/alz.13016
22. Tahami Monfared AA, Byrnes MJ, White LA, Zhang Q. The humanistic and economic burden of Alzheimer’s disease. Neurol Ther. 2022;11(2):525-551. doi: 10.1007/s40120-022-00335-x
23. Lin PJ, Zhong Y, Fillit HM, Chen E, Neumann PJ. Medicare expenditures of individuals with Alzheimer’s disease and related dementias or mild cognitive impairment before and after diagnosis. J Am Geriatr Soc. 2016;64(8):1549-1557. doi: 10.1111/jgs.14227
24. Stites SD, Harkins K, Rubright JD, Karlawish J. Relationships between cognitive complaints and quality of life in older adults with mild cognitive impairment, mild Alzheimer disease dementia, and normal cognition. Alzheimer Dis Assoc Disord. 2018;32(4):276-283. doi: 10.1097/WAD.0000000000000262
25. Ito K, Chapman R, Pearson SD, Tafazzoli A, Yaffe K, Gurwitz JH. Evaluation of the cost-effectiveness of drug treatment for Alzheimer disease in a simulation model that includes caregiver and societal factors. JAMA Netw Open. 2021;4(10):e2129392. doi: 10.1001/jamanetworkopen.2021.29392
26. Tahami Monfared AA, Ye W, Sardesai A, et al. estimated societal value of lecanemab in patients with early Alzheimer’s disease using simulation modeling. Neurol Ther. 2023;12(3):795-814. doi: 10.1007/s40120-023-00460-1
27. Bateman RJ, Smith J, Donohue MC, et al. Two phase 3 trials of gantenerumab in early Alzheimer’s disease. N Engl J Med. 2023;389(20):1862-1876. doi: 10.1056/NEJMoa2304430
28. Bateman RJ, Cummings J, Schobel S, et al. Gantenerumab: an anti-amyloid monoclonal antibody with potential disease-modifying effects in early Alzheimer’s disease. Alzheimers Res Ther. 2022;14(1):178. doi: 10.1186/s13195-022-01110-8
29. Research Data Assistance Center (ResDAC). CMS cell size suppression policy. https://resdac.org/articles/cms-cell-size-suppression-policy#:~:text=containing%20a%20value%20of%201,other%20reported%20cells%20or%20information. Accessed 8 January 2024
30. Research Data Assistance Center (ResDAC). Identifying Medicare managed care beneficiaries from the Master Beneficiary Summary or denominator files. June 22, 2021. https://resdac.org/articles/identifying-medicare-managed-care-beneficiaries-master-beneficiary-summary-or-denominator. Accessed 5 December 2023.
31. Wise-Brown A, Markham-Coultes K, Gonzales-Rojas Y, et al. Baseline racial and ethnic characteristics of United States participants enrolled in the Phase III GRADUATE trials of gantenerumab for early AD. Presented at: Addressing Health Disparities; June 21-22, 2022; Washington, DC and virtual. https://medically.gene.com/global/en/unrestricted/neuroscience/AAAHD-2022/aaahd-2022-poster-wise-brown-baseline-racial-and-ethnic.html. Accessed 8 January 2024
32. Ochieng N, Fuglesten Biniek J, Freed M, Damico A, Neuman T. Medicare Advantage in 2023: enrollment update and key trends. KFF Health News. August 9, 2023. https://www.kff.org/medicare/issue-brief/medicare-advantage-in-2023-enrollment-update-and-key-trends/. Accessed 5 December 2023.
33. Mattke S, Jun H, Chen E, Liu Y, Becker A, Wallick C. Expected and diagnosed rates of mild cognitive impairment and dementia in the U.S. Medicare population: observational analysis. Alzheimers Res Ther. 2023;15(1):128. doi: 10.1186/s13195-023-01272-z
34. Khandker RK, Ritchie CW, Black CM, et al. Multi-national, cross-sectional survey of healthcare resource utilization in patients with all stages of cognitive impairment, analyzed by disease severity, country, and geographical region. J Alzheimers Dis. 2020;75(4):1141-1152. doi: 10.3233/JAD-190760
35. Wallick C, Nunag D, Zuk E, Davis M, Majda T. Long-term trends in healthcare resource use for US Medicare patients following Alzheimer’s disease diagnosis. Presented at Academy of Managed Care Pharmacy (AMCP) 2023; March 21-24, 2023; San Antonio, TX. https://medically.gene.com/content/dam/pdmahub/restricted/neurology/amcp-2023/AMCP-2023-poster-wallick-long-term-trends-in-healthcare-resource.pdf. Accessed 8 January 2024.
36. Abbass IM, Choi D, Wallick C, Assunção SS. Trends in healthcare resource use preceding diagnosis of Alzheimer’s disease dementia. Int J Alzheimers Dis. 2023;2023:8154701. doi: 10.1155/2023/8154701
37. Jacobson M, Ferido P, Zissimopoulos J. Health care utilization before and after a dementia diagnosis in Medicare Advantage versus traditional Medicare. J Am Geriatr Soc. Published online 5 September 2023. doi:10.1111/jgs.18570
38. Desai U, Kirson NY, Ye W, Mehta NR, Wen J, Andrews JS. Trends in health service use and potentially avoidable hospitalizations before Alzheimer’s disease diagnosis: a matched, retrospective study of US Medicare beneficiaries. Alzheimers Dement (Amst). 2019;11:125-135. doi: 10.1016/j.dadm.2018.12.005
39. Seleri Assunção S, Brangman SA, Henderson JN, et al. ALUMNI AD study design: evaluation of subcutaneous gantenerumab in historically underrepresented US populations with early symptomatic Alzheimer’s disease. Abstract presented at: The International Conference on Alzheimer’s and Parkinson’s Diseases and Related Neurological Disorders Annual Meeting; March 31, 2023; Gothenburg, Sweden.
40. Pisu M, Martin RC, Shan L, et al. Dementia care in diverse older adults in the U.S. deep south and the rest of the United States. J Alzheimers Dis. 2021;83(4):1753-1765. doi: 10.3233/JAD-210240
41. Rashid N, Wetmore JB, Irfan M, Abler V. Economic evaluation of healthcare resource utilization and costs for newly diagnosed dementia-related psychosis. Geriatrics (Basel). 2022;7(2):29. doi: 10.3390/geriatrics7020029
© Serdi 2024