A.F. Silva1,2,3, R.M. Silva1,2, E. Murawska-Ciałowicz4, G. Zurek5, N. Danek4, M. Cialowicz6, J. Carvalho7, F.M. Clemente1,2,8
1. Escola Superior Desporto e Lazer, Instituto Politécnico de Viana do Castelo, Rua Escola Industrial e Comercial de Nun’Álvares, 4900-347 Viana do Castelo, Portugal; 2. Research Center in Sports Performance, Recreation, Innovation and Technology (SPRINT), 4960-320 Melgaço, Portugal; 3. The Research Centre in Sports Sciences, Health Sciences and Human Development (CIDESD), 5000-801 Vila Real, Portugal; 4. Department of Physiology and Biochemistry, Wroclaw University of Health and Sport Sciences, 51-612 Wroclaw, Poland; 5. Department of Biostructure, Wroclaw University of Health and Sport Sciences, 51-612 Wrocław, Poland; 6. Department of Physiotherapy in Interventional Medicine and Oncology, Wroclaw University of Health and Sport Science, 51-612 Wrocław, Poland; 7. High Performance Brain, Corkstraat 46, 3047 AC, Rotterdam, Netherlands; 8. Gdansk University of Physical Education and Sport, 80-336, Gdańsk, Poland
Corresponding Author: Rui Miguel Silva, Escola Superior de Desporto e Lazer – Instituto Politécnico de Viana do Castelo, Complexo Desportivo e Lazer Comendador Rui Solheiro Monte de Prado, 4960-320 Melgaço, Tel.. +351 258 809 678, rui.s@ipvc.pt
J Prev Alz Dis 2024;3(11):693-700
Published online January 23, 2024, http://dx.doi.org/10.14283/jpad.2024.17
Abstract
INTRODUCTION: The present scoping review focused on: i) which apps were previously studied; ii) what is the most common frequency for implementing cognitive training; and iii) what cognitive functions the interventions most focus on.
METHODS: PRISMA guidelines were followed, and the search was conducted on Web of Science, PsycInfo, Cochrane, and Pubmed. From 1733 studies found, 34 were included.
RESULTS: it was highlighted the necessity for forthcoming investigations to tackle the methodical restrictions and disparities in the domain.
DISCUSSION: great diversity in intervention protocols was found. Incorporating evaluations of physical fitness in conjunction with cognitive evaluations can offer a more all-encompassing comprehension of the impacts of combined interventions. Furthermore, exploring the efficacy of cognitive training applications requires additional scrutiny, considering individual variances and practical outcomes in real-life settings.
Key words: Cognition, mild cognitive impairment, Alzheimer’s disease, mobile tech, cognitive training.
Introduction
Dementia, a progressive neurocognitive disorder, is characterized by the deterioration of cognitive functions encompassing language, memory, perception, and thought, persisting until an individual’s passing (1). A diagnosis of dementia is made when two or more of these core mental functions are impaired (2). As global populations continue to age, the prevalence of dementia is increasing, with current estimates indicating that approximately 44 million people are affected by this chronic neurological condition (3). Projections indicate that by 2050, this chronic neurological condition will afflict approximately 14,298,671 individuals in the European Union (EU) and 18,846,286 in the broader European region (4). Notably, among the elderly population, 50% to 80% of individuals aged 70 or older, scoring within the normal range on cognitive tests, report perceived cognitive decline (2).
According to the National Institute of Aging-Alzheimer’s Association (NIA-AA) (5), Alzheimer’s disease, the most common form of dementia, exhibits a symptomatology that spans six progressive stages, reflecting the increasing severity of the condition. In light of this, there appears to be a window of opportunity for delaying the development of dementia, especially considering the absence of a known cure. Recognizing this, the World Health Organization (WHO) has designated dementia as a public health priority. Between a healthy state and the diagnosis of dementia lies a condition known as mild cognitive impairment (MCI). Individuals with MCI also experience cognitive declines, but they typically maintain their independence and continue to function well in everyday life (6, 7). These cognitive declines typically affect processing speed, executive functions, memory, and visuospatial abilities (8).
In response, the concept of cognitive training and stimulation has evolved. Cognitive training involves repetitive practice targeting specific cognitive functions with standardized tasks of varying difficulty, while cognitive stimulation takes a non-specific approach, emphasizing diverse activities and social interaction (9, 10). Fortunately, interventional studies on both cognitive training and cognitive rehabilitation have shown promising effects in improving cognitive function among individuals with MCI (11–14). This is attributed to the human brain’s capacity for neuroplasticity, which refers to the brain’s ability to undergo morphological changes in response to environmental stimuli (15). Consequently, the brain can adapt and compensate for cognitive changes by reinforcing existing connections or forming new ones. This capacity persists throughout one’s lifespan and can be influenced by various factors, including genetics, education level, occupation, socioeconomic status, physical health, lifestyle choices, and mental engagement (16).
Technology’s ubiquity, even among older adults, has resulted in numerous smartphone and/or web-based applications (Apps) designed to assist individuals with MCI. Within the realm of cognitive training and stimulation, studies have identified promising strategies for preserving cognitive function in healthy older adults and individuals with MCI (17). Comparing technology-based interventions with traditional programs has shown the former to yield overall superior outcomes in enhancing cognitive function and quality of life (18–20). Computerized cognitive training’s beneficial effects endure in individuals with preserved cognitive function, both short-term and long-term (21). Additionally, cognitive training has been recognized as one of the most effective interventions, as it has the potential to decelerate the progression of cognitive decline in individuals with dementia in rehabilitation settings (22, 23). However, while technology-based cognitive training interventions hold promise, a systematic review revealed that their effectiveness remains inconsistent, primarily due to variations in study design (24).
As there is currently no known cure for dementia, prioritizing the enhancement of preventive strategies to delay its onset and slow down cognitive decline has become imperative (12). Furthermore, the pharmaceutical treatments currently used for dementia do not consistently produce significant modifications in the progression of the disease (25). As a result, the cognitive training approach has garnered considerable attention (26, 27). For those reasons, the present scoping review had three main objectives, which were to describe the apps previously studied, investigate the most common frequency for implementing cognitive training, and assess the primary cognitive functions targeted by these interventions.
Methods
Protocol and registration
This scoping review followed the PRISMA 2020 guidelines (28) and also took into consideration the recommendations for the scoping reviews checklist (PRISMA-ScR) (29). The protocol was pre-registered in the Open Science Framework (OSF) (associated with the project: https://osf.io/e4znf/).
Eligibility criteria
Studies published in peer-reviewed journals, including those with the status of “in press” or “ahead-of-print”, were considered. No limitations to date were established. Only English studies were included. The eligibility criteria were determined following the PECOS approach (population, exposure, comparator, outcome, study design): (i) population: inclusion of cognitively healthy older adults and older adults with cognitive impairment (including Alzheimer’s, dementia, or other cognitive declines); (ii) exposure: participants exposed to cognitive training, including technology-based methods (web-based apps and smartphone apps), as well as manual games/tasks; (iii) comparator: individuals not exposed to medication; (iv) outcome(s): assessment of the implemented games/tasks and the specific cognitive functions targeted; and v) study design: consideration of both observational studies and interventions.
Information sources
The present study used the following databases Web of Science, PsycInfo, Cochrane, and Pubmed. After performing the protocol registration, the searches were conducted on the same day. In addition to the database searches, a manual search was performed on: (i) the reference lists of included studies to identify potentially relevant titles and checking the abstract for pertinent inclusion criteria and, if necessary, the full text; (ii) snowballing citation tracking, preferably in Web of Science; and (iii) consultation of two external experts (as recognized by Expertscape: https://expertscape.com/ex/soccer). Finally, errata/retractions will also be analyzed for any articles that were included (30).
Search strategy
The search strategy applied the Boolean operators AND/OR. No filters or limitations were used (e.g., date; study design) to maximize the chances of identifying appropriate studies (31). The main search strategy was as follows:
[All fields/Full text] “cogni* rehabilitation” OR “cogni* stimulation” OR “cogni* train*”
AND
[All fields/Full text] Alzheimer OR “cogni* decline” OR “cogni* impair*” OR MCI OR dementia
AND
[All fields/Full text] software* OR “mobile app*” OR “smartphone app*” OR “smartphone applications*” OR “mobile tech*” OR “assistive tech*”
Table 1 presents the codes used for each database.
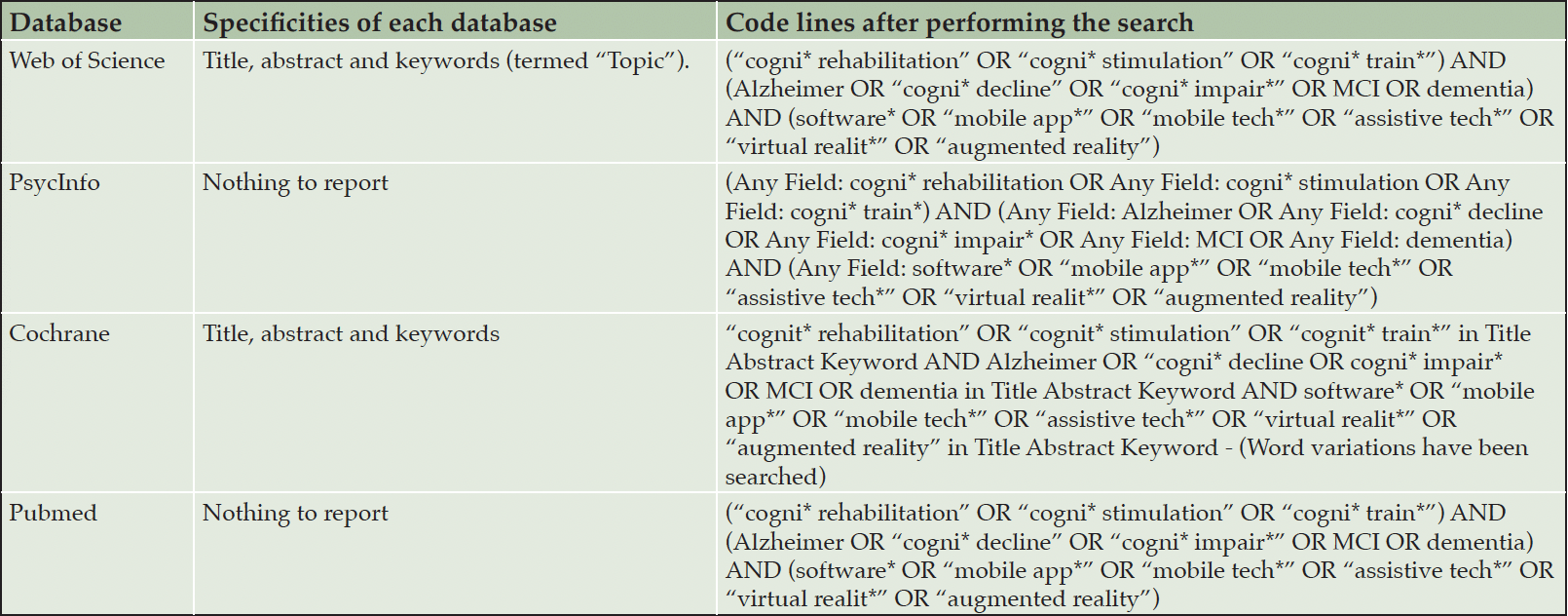
Table 1. Resulting code lines for each database (preliminary tests, before registering the protocol; no records were retrieved at this point)
Selection process
Two of the authors (AFS and JC) independently retrieved records, which included titles and abstracts. These same authors then conducted an independent screening of the collected full-text articles. Any disagreements between the two authors were resolved through joint reanalysis. In cases where a consensus could not be reached, a third author (FMC) made the final decision. Whenever necessary, all co-authors provided their input on any uncertainties that arose during the selection process to support the final decision. Mendeley software (Elsevier) was utilized for managing records, including the automated or manual removal of duplicates.
Data collection
The primary author (AFS) initially conducted the data collection process. Subsequently, two co-authors (ND and MC) performed a double-check to ensure the accuracy and completeness of the collected data. To facilitate this process, a specially designed Microsoft® Excel datasheet was created and used for data extraction, capturing essential information. The Excel datasheet is available in the supplementary material for reference. In cases where relevant data were missing from the full text, the primary author (AFS) directly contacted the corresponding author of the document via email and/or ResearchGate to obtain the necessary information.
Data items
In this section, the data items considered for the review are detailed. These included participant characteristics such as age, sex, cognitive training frequency, and the stage of dementia. Context-related information, specifically the number of sessions under consideration, was also captured. The main outcomes of interest encompassed the tests that were implemented and the cognitive functions assessed. Comparisons were made with other cognitive interventions, which might have involved medical or musical interventions, or a control group undergoing any form of intervention. Additionally, supplementary information was recorded, including the publication date, funding details, and any competing interests disclosed by the studies under review.
Study risk of bias assessment
Two authors (EMC and JC) independently assessed the risk of bias. In the case of disagreements, both reanalyzed the process. In the case of no subsequent consensus being reached, a third author (AFS) made the final decision. The Risk of Bias Assessment Tool for Nonrandomized Studies (RoBANS) was used to assess the risk of bias in the included studies (32). The scale has shown moderate reliability, feasibility, and validity (32). The tool comprises six domains: (i) the selection of participants; (ii) confounding variables; (iii) the measurement of exposure; (iv) the blinding of the outcome assessments; (v) incomplete outcome data; and (vi) selective outcome reporting. The domains are classified as ‘low’, ‘high’, and ‘unclear’ risk of bias.
Data management and synthesis methods
A narrative synthesis was performed, including data summaries presenting numbers and percentages for predefined data items. To provide an overview of the existing research landscape and emphasize areas requiring further investigation, an evidence-gap map (EGM) was developed. The EGM serves as a visual representation to convey the existing evidence and highlight current research gaps (33–35). Table 2 presents a provisional EGM.
MCI: mild cognitive impairment; AD: Alzheimer’s disease
Results
Literature search
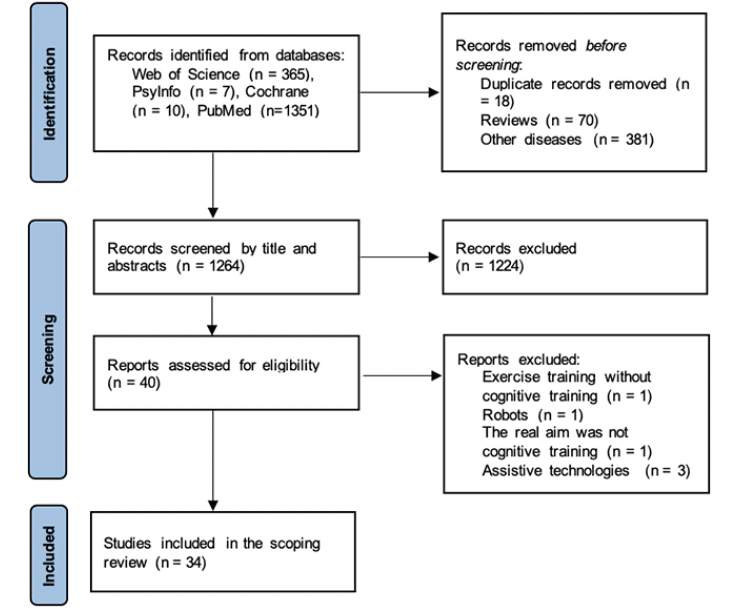
The literature search showed the existence of 1733 studies. From those, 469 were excluded as they were duplicated (n = 18), others were reviews (n = 70), and the majority of them were from other diseases such as Parkinson’s, Schizophrenia, cancer, and strokes, among others (n = 381). In screening the title and abstract, 1224 were excluded as they focused on other rehabilitation strategies/protocols or did not have any technology implemented. Moreover, other articles did not include interventions and just presented an app with only one applied session. In the end, 40 studies were assessed for eligibility by full-text analysis. Finally, 34 articles met the criteria for inclusion. A flowchart of the literature search and selection process is presented in Figure 1.
Apps used
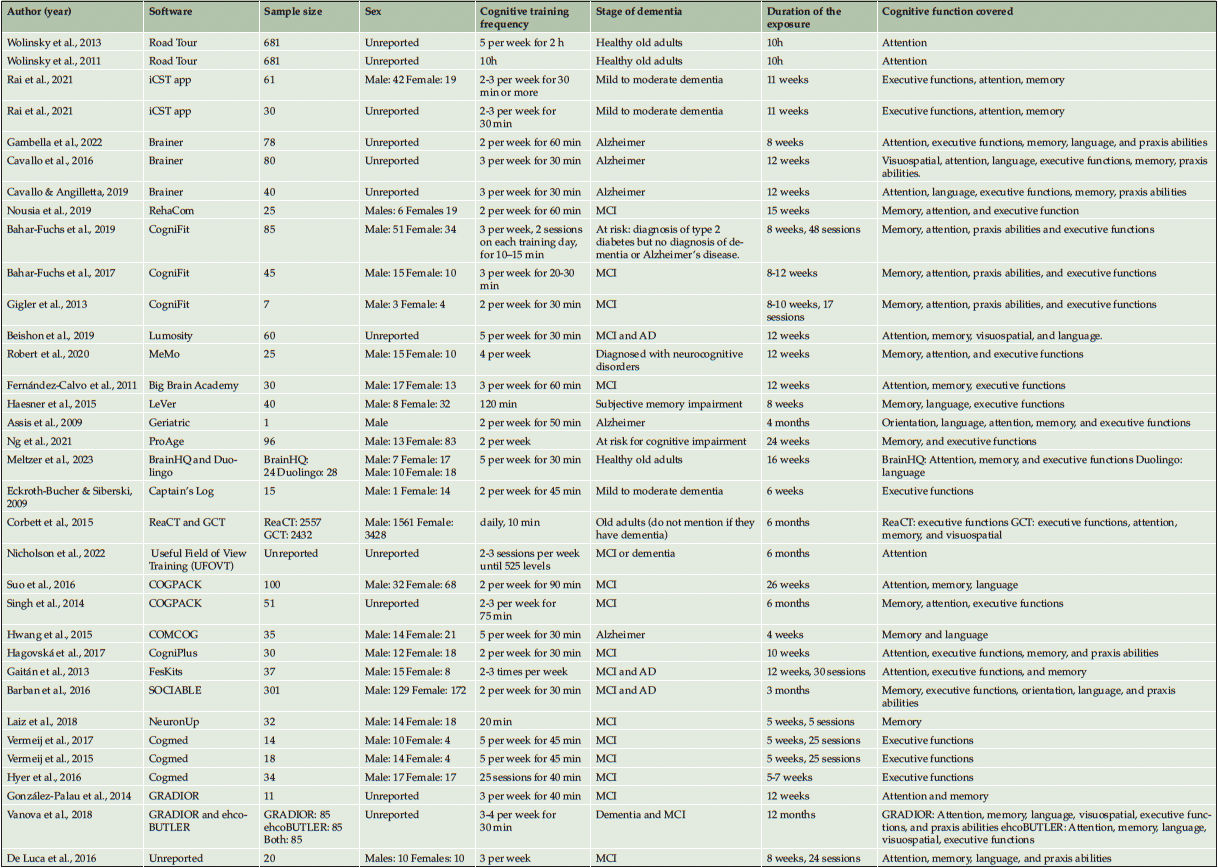
In the 34 studies included, 27 apps were used. The Brainer, CogniFit, and Cogmed were the most used apps with 3 studies published for each app, followed by Road Tour, iCST app, COGPACK, and GRADIOR with 2 studies published for each app. The other studies had only one study published.
Study characteristics
The studies varied greatly in the characteristics of the sample, with studies from just one subject (Assis et al., 2010) including up to 2557 subjects (36). Nevertheless, from the 34 studies included, only 4 studies integrated 100 or more subjects in their samples. The other studies presented a mean of approximately 39 subjects.
Most of the studies (65%, corresponding to 22 studies) included both sexes in the interventions, and only one study included a very small sample size, consisting of one male. However, the remaining 32% of the studies (11 studies) did not describe the sex of the subjects included in the sample. They only mentioned that they were old adults or adults. Considering the stage of dementia, 3 studies did not include any dementia or impairment (37–39), and one study did not mention any information regarding the state of mental and cognitive health (36), and 2 other studies analyzed subjects at risk of dementia (40, 41). The remaining 28 studies included subjects with mild to moderate impairment or even with a diagnosis of Alzheimer’s disease.
Types of interventions
The duration of the interventions was quite varied, with a range of 20 (42) to 600 (37, 38) minutes per week of practice in the technologies, with a range of 10 (36, 40) to 240 minutes per session. Also, the frequency varied between 2 (18, 41, 43–53) to every day (36) (of all the studies that explicitly mentioned the frequency) and lasted from just one week with 10 intensive hours (37, 38) to 12 months (54).
Cognitive functions covered
Considering all studies, the cognitive functions covered were memory (79.4%), attention (73.5%), executive functions (76.5%) language (35.3%), praxis abilities (26.5%), visuospatial (11.8%), and orientation (5.9%). The cognitive functions of memory, executive functions, and attention stand out clearly with 27, 26, and 25 studies, respectively.
Discussion
This scoping review aimed to provide insights into the landscape of cognitive training apps, their implementation frequencies, durations, and the primary cognitive functions they target. While our analysis shed light on these aspects, it is equally important to delve into the distinctive challenges and nuances associated with the use of these apps in the context of older adults living with cognitive impairment. This expanded discussion aims to address these specific considerations.
Most of the protocols and interventions used to assess cognitive functions focused mainly on memory, executive functions, and attention tasks. Among others, the most used apps during cognitive function training interventions were the Brainer, CogniFit, and Cogmed apps. Although the majority of these studies assessed cognitive impairments through the use of questionnaires, such as the mild cognitive impairment Peterson criteria (55), the Mini-mental state (56), the Alzheimer’s disease assessment scale (57), and the Cornell scale for depression in dementia (58), the overall studies did not categorize their samples according to each cognitive impairment that was assessed at the baseline. Different cognitive impairments show differentiated characteristics and may respond differently to cognitive training interventions (12). Without accounting for these differences, it may be challenging to draw accurate conclusions about the effectiveness of apps in addressing specific cognitive impairments.
The wide variation in intervention protocols, such as the type, duration, intensity, and frequency of cognitive training, may lead to inconsistent findings and limit the ability to compare and generalize the results. For instance, while the overall studies implemented an average of two sessions per week (18, 45, 47, 51, 59, 60), one study implemented cognitive training sessions on all days of week (61). Also, the duration of cognitive training was widely widespread, from a minimum of 20 minutes to a maximum of 600 minutes per week (37, 38, 42). Additionally, it is worth noting that 10 of the 34 studies analyzed (37, 51, 60, 62–68) did not specify the sex composition of their samples. Neglecting to provide information regarding the sex distribution within these studies may introduce biases that hinder the comprehensive understanding of their findings and impede meaningful comparisons with the existing evidence.
Moreover, there are other challenges associated with the use of these apps that must be addressed. For instance, as individuals age, there is a natural decline in cognitive function, including a decrease in processing speed (69). This highlights the importance of considering the baseline cognitive abilities and age-related changes when implementing cognitive training apps for older adults with cognitive impairment. The literature suggests that basic numerical skills are generally preserved in healthy aging (70). However, individuals with MCI show difficulties in understanding numerical and arithmetic tasks (71). This finding indicates that when designing cognitive training apps, it’s essential to distinguish between age-related changes and impairments specific to cognitive conditions such as MCI. Importantly, the impact of cognitive training apps may vary based on individual experience and perceptions (72). Given that, it is imperative to match the technology to older adults’ needs and assess their experiences. Lastly, although early detection of cognitive decline can aid research and clinical intervention, it poses adherence challenges to the use of such smartphone/web-based cognitive training apps (73).
The present scoping review has certain limitations that warrant attention. One limitation pertains to the employed search strategy and criteria for article eligibility. We implemented a search strategy that was unanimously agreed upon by our team of authors and experts. Nonetheless, it is important to acknowledge that like any search strategy, it may not have captured all relevant articles. Nonetheless, we made efforts to minimize this potential bias by employing a comprehensive search strategy. Our research methodology was conducted by the PRISMA statement, ensuring a comprehensive and rigorous approach that surpasses traditional scoping reviews. A previous systematic review which adroitly harnessed between-study differences to investigate factors such as dosage effects and target-specific outcomes, offers a noteworthy path forward in this domain (24). The mentioned systematic analysis highlighted the existence of modest yet discernible efficacy of computerized cognitive training in enhancing cognitive performance among healthy older adults, all while delineating the influential role played by design factors (24). Although this approach was out of the scope of this scoping review, our presentation of results was guided by a rationale that prioritized the analysis of methodological approaches utilized in the original studies, expanding beyond the sole consideration of primary outcomes reported.
Further research is essential to determine which cognitive training interventions work best for different populations and cognitive domains. Standardizing protocols and comparing various approaches, such as computer-based training, gamified interventions, and combined interventions with physical training, can help address these questions. Future research should identify factors influencing responses to cognitive training, including age, baseline cognitive abilities, genetic predispositions, and individual differences. Moreover, longitudinal studies could provide insights into the long-term effects of combined cognitive and physical interventions on cognition, fitness, and well-being. Future research may explore personalized cognitive interventions through apps and physical training, tailoring exercises based on participants’ cognitive abilities and fitness levels.
Conclusion
This scoping review highlights the multifaceted challenges in using cognitive training apps for older adults with cognitive impairment. Categorizing participants based on cognitive impairment type, addressing intervention heterogeneity, and considering age-related changes are crucial for effective app-based interventions. Moreover, recognizing individual experiences and needs, assessing adherence issues, and adapting technology accordingly is essential. Future research should focus on standardizing protocols, understanding population-specific responses, and exploring personalized cognitive interventions through apps and physical training for improved cognitive health in older adults.
Funding: This work was supported by the Erasmus+ Programme (ERASMUS) [ERASMUS-SPORT-2022-SCP], the European Commission [101090386 — Fit4Alz]. Open access funding provided by FCT|FCCN (b-on).
Consent Statement: Consent was not necessary to conduct this scoping review.
Conflicts of Interest: All authors declare that they have no conflicts of interest.
Open Access: This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
References
1. Dubois B. The Emergence of a New Conceptual Framework for Alzheimer’s Disease. Journal of Alzheimer’s Disease. 2018;62(3):1059-1066. doi:10.3233/JAD-170536
2. Brown A, O’Connor S. Mobile health applications for people with dementia: a systematic review and synthesis of qualitative studies. Inform Health Soc Care. 2020;45(4):343-359. doi:10.1080/17538157.2020.1728536
3. Alzheimer’s Disease International. World Alzheimer Report 2015: The Global Impact of Dementia – An Analysis of Prevalence, Incidence, Cost and Trends. Alzheimer’s Disease International; 2015.
4. Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: A systematic review and metaanalysis. Alzheimer’s & Dementia. 2013;9(1):63-75.e2. doi:10.1016/j.jalz.2012.11.007
5. O’Connor S. Virtual Reality and Avatars in Health care. Clin Nurs Res. 2019;28(5):523-528. doi:10.1177/1054773819845824
6. National Collaborating Centre for Mental Health. Dementia: A NICE-SCIE Guideline on Supporting People with Dementia and Their Carers in Health and Social Care.; 2007.
7. Ge S, Zhu Z, Wu B, McConnell ES. Technology-based cognitive training and rehabilitation interventions for individuals with mild cognitive impairment: a systematic review. BMC Geriatr. 2018;18(1):213. doi:10.1186/s12877-018-0893-1
8. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. International Journal of Surgery. 2010;8(5):336-341. doi:10.1016/j.ijsu.2010.02.007
9. Raggi A, Tasca D, Ferri R. A brief essay on non-pharmacological treatment of Alzheimer’s disease. Rev Neurosci. 2017;28(6):587-597. doi:10.1515/revneuro-2017-0002
10. Clare L, Woods B. Cognitive rehabilitation and cognitive training for early-stage Alzheimer’s disease and vascular dementia. In: Clare L, ed. Cochrane Database of Systematic Reviews. John Wiley & Sons, Ltd; 2003. doi:10.1002/14651858.CD003260
11. Jean L, Bergeron MÈ, Thivierge S, Simard M. Cognitive Intervention Programs for Individuals With Mild Cognitive Impairment: Systematic Review of the Literature. The American Journal of Geriatric Psychiatry. 2010;18(4):281-296. doi:10.1097/JGP.0b013e3181c37ce9
12. Gates NJ, Sachdev PS, Fiatarone Singh MA, Valenzuela M. Cognitive and memory training in adults at risk of dementia: A Systematic Review. BMC Geriatr. 2011;11(1):55. doi:10.1186/1471-2318-11-55
13. Reijnders J, van Heugten C, van Boxtel M. Cognitive interventions in healthy older adults and people with mild cognitive impairment: A systematic review. Ageing Res Rev. 2013;12(1):263-275. doi:10.1016/j.arr.2012.07.003
14. Tsantali E, Economidis D, Rigopoulou S. Testing the Benefits of Cognitive Training vs. Cognitive Stimulation in Mild Alzheimer’s Disease: A Randomised Controlled Trial. Brain Impairment. 2017;18(2):188-196. doi:10.1017/BrImp.2017.6
15. Shaffer J. Neuroplasticity and Clinical Practice: Building Brain Power for Health. Front Psychol. 2016;7. doi:10.3389/fpsyg.2016.01118
16. Sampedro-Piquero P, Begega A. Environmental Enrichment as a Positive Behavioral Intervention Across the Lifespan. Curr Neuropharmacol. 2017;15(4):459-470. doi:10.2174/1570159X14666160325115909
17. Zhang H, Huntley J, Bhome R, et al. Effect of computerised cognitive training on cognitive outcomes in mild cognitive impairment: a systematic review and meta-analysis. BMJ Open. 2019;9(8):e027062. doi:10.1136/bmjopen-2018-027062
18. Hagovská M, Dzvoník O, Olekszyová Z. Comparison of two cognitive training programs with effects on functional activities and quality of life. Res Gerontol Nurs. 2017;10(4):172-180.
19. Faucounau V, Wu YH, Boulay M, De Rotrou J, Rigaud AS. Cognitive intervention programmes on patients affected by Mild Cognitive Impairment: A promising intervention tool for MCI? J Nutr Health Aging. 2010;14(1):31-35. doi:10.1007/s12603-010-0006-0
20. Ball K, Berch DB, Helmers KF, et al. Effects of Cognitive Training Interventions With Older Adults. JAMA. 2002;288(18):2271. doi:10.1001/jama.288.18.2271
21. ten Brinke LF, Best JR, Crockett RA, Liu-Ambrose T. The effects of an 8-week computerized cognitive training program in older adults: a study protocol for a randomized controlled trial. BMC Geriatr. 2018;18(1):31. doi:10.1186/s12877-018-0730-6
22. Hill NTM, Mowszowski L, Naismith SL, Chadwick VL, Valenzuela M, Lampit A. Computerized Cognitive Training in Older Adults With Mild Cognitive Impairment or Dementia: A Systematic Review and Meta-Analysis. Am J Psychiatry. 2017;174(4):329-340. doi:10.1176/appi.ajp.2016.16030360
23. Amieva H, Robert PH, Grandoulier AS, et al. Group and individual cognitive therapies in Alzheimer’s disease: the ETNA3 randomized trial. Int Psychogeriatr. 2016;28(5):707-717. doi:10.1017/S1041610215001830
24. Lampit A, Hallock H, Valenzuela M. Computerized cognitive training in cognitively healthy older adults: a systematic review and meta-analysis of effect modifiers. PLoS Med. 2014;11(11):e1001756. doi:10.1371/journal.pmed.1001756
25. Waite L. Treatment for Alzheimer’s disease: has anything changed? Aust Prescr. 2015;38(2):60-63. doi:10.18773/austprescr.2015.018
26. Raggi A, Iannaccone S, Marcone A, et al. The effects of a comprehensive rehabilitation program of Alzheimer’s disease in a hospital setting. Behavioural Neurology. 2007;18:1-6.
27. Panerai S, Tasca D, Musso S, et al. Group Intensive Cognitive Activation in Patients with Major or Mild Neurocognitive Disorder. Front Behav Neurosci. 2016;10. doi:10.3389/fnbeh.2016.00034
28. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. Published online March 2021:n71. doi:10.1136/bmj.n71
29. Tricco AC, Lillie E, Zarin W, et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann Intern Med. 2018;169(7):467-473. doi:10.7326/M18-0850
30. Higgins J, Thomas J. Cochrane Handbook for Systematic Reviews of Interventions. Cochrane; 2021.
31. Wong SS, Wilczynski NL, Haynes RB. Developing optimal search strategies for detecting clinically sound treatment studies in EMBASE. Journal of the Medical Library Association. 2006;94(1):41-47.
32. Kim SY, Park JE, Lee YJ, et al. Testing a tool for assessing the risk of bias for nonrandomized studies showed moderate reliability and promising validity. J Clin Epidemiol. 2013;66(4):408-414.
33. Schuller-Martínez B, Meza N, Pérez-Bracchiglione J, Ariel Franco JV, Loezar C, Madrid E. Graphical representation of the body of the evidence: the essentials for understanding the evidence gap map approach. Medwave. 2021;21(03):e8164-e8164. doi:10.5867/medwave.2021.03.8164
34. Snilstveit B, Vojtkova M, Bhavsar A, Stevenson J, Gaarder M. Evidence & Gap Maps: A tool for promoting evidence informed policy and strategic research agendas. J Clin Epidemiol. 2016;79:120-129. doi:10.1016/j.jclinepi.2016.05.015
35. Miake-Lye IM, Hempel S, Shanman R, Shekelle PG. What is an evidence map? A systematic review of published evidence maps and their definitions, methods, and products. Syst Rev. 2016;5(1):28. doi:10.1186/s13643-016-0204-x
36. Corbett A, Owen A, Hampshire A, et al. The Effect of an Online Cognitive Training Package in Healthy Older Adults: An Online Randomized Controlled Trial. J Am Med Dir Assoc. 2015;16(11):990-997. doi:10.1016/j.jamda.2015.06.014
37. Wolinsky FD, Vander Weg MW, Howren MB, Jones MP, Dotson MM. A randomized controlled trial of cognitive training using a visual speed of processing intervention in middle aged and older adults. PLoS One. 2013;8(5):e61624. doi:10.1371/journal.pone.0061624
38. Wolinsky FD, Vander Weg MW, Howren MB, et al. Protocol for a randomised controlled trial to improve cognitive functioning in older adults: the Iowa Healthy and Active Minds Study. BMJ Open. 2011;1(2):e000218-e000218. doi:10.1136/bmjopen-2011-000218
39. Meltzer JA, Kates Rose M, Le AY, et al. Improvement in executive function for older adults through smartphone apps: a randomized clinical trial comparing language learning and brain training. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2023;30(2):150-171. doi:10.1080/13825585.2021.1991262
40. Bahar-Fuchs A, Barendse MEA, Bloom R, et al. Computerized Cognitive Training for Older Adults at Higher Dementia Risk due to Diabetes: Findings From a Randomized Controlled Trial. J Gerontol A Biol Sci Med Sci. 2020;75(4):747-754. doi:10.1093/gerona/glz073
41. Ng PEM, Nicholas SO, Wee SL, et al. Implementation and effectiveness of a multi-domain program for older adults at risk of cognitive impairment at neighborhood senior centres. Sci Rep. 2021;11(1):3787. doi:10.1038/s41598-021-83408-5
42. Mendoza Laiz N, Del Valle Díaz S, Rioja Collado N, Gomez-Pilar J, Hornero R. Potential benefits of a cognitive training program in mild cognitive impairment (MCI). Restor Neurol Neurosci. 2018;36(2):207-213. doi:10.3233/RNN-170754
43. Rai HK, Schneider J, Orrell M. An Individual Cognitive Stimulation Therapy App for People with Dementia and Carers: Results from a Feasibility Randomized Controlled Trial (RCT). Clin Interv Aging. 2021;Volume 16:2079-2094. doi:10.2147/CIA.S323994
44. Rai HK, Schneider J, Orrell M. An Individual Cognitive Stimulation Therapy App for People With Dementia and Their Carers: Protocol for a Feasibility Randomized Controlled Trial. JMIR Res Protoc. 2021;10(4):e24628. doi:10.2196/24628
45. Nousia A, Martzoukou M, Siokas V, et al. Beneficial effect of computer-based multidomain cognitive training in patients with mild cognitive impairment. Appl Neuropsychol Adult. 2021;28(6):717-726. doi:10.1080/23279095.2019.1692842
46. Gambella E, Margaritini A, Benadduci M, et al. An integrated intervention of computerized cognitive training and physical exercise in virtual reality for people with Alzheimer’s disease: The jDome study protocol. Front Neurol. 2022;13. doi:10.3389/fneur.2022.964454
47. Gigler KL, Blomeke K, Shatil E, Weintraub S, Reber PJ. Preliminary evidence for the feasibility of at-home online cognitive training with older adults. Gerontechnology. 2013;12(1). doi:10.4017/gt.2013.12.1.007.00
48. Eckroth-Bucher M, Siberski J. Preserving cognition through an integrated cognitive stimulation and training program. Am J Alzheimers Dis Other Demen. 2009;24(3):234-245. doi:10.1177/1533317509332624
49. Suo C, Singh MF, Gates N, et al. Therapeutically relevant structural and functional mechanisms triggered by physical and cognitive exercise. Mol Psychiatry. 2016;21(11):1633-1642. doi:10.1038/mp.2016.19
50. Fiatarone Singh MA, Gates N, Saigal N, et al. The Study of Mental and Resistance Training (SMART) Study—Resistance Training and/or Cognitive Training in Mild Cognitive Impairment: A Randomized, Double-Blind, Double-Sham Controlled Trial. J Am Med Dir Assoc. 2014;15(12):873-880. doi:10.1016/j.jamda.2014.09.010
51. Nicholson JS, Hudak EM, Phillips CB, et al. The Preventing Alzheimer’s with Cognitive Training (PACT) randomized clinical trial. Contemp Clin Trials. 2022;123:106978. doi:10.1016/j.cct.2022.106978
52. Gaitán A, Garolera M, Cerulla N, Chico G, Rodriguez-Querol M, Canela-Soler J. Efficacy of an adjunctive computer-based cognitive training program in amnestic mild cognitive impairment and Alzheimer’s disease: A single-blind, randomized clinical trial. Int J Geriatr Psychiatry. 2013;28(1):91-99.
53. Barban F, Annicchiarico R, Pantelopoulos S, et al. Protecting cognition from aging and Alzheimer’s disease: a computerized cognitive training combined with reminiscence therapy. Int J Geriatr Psychiatry. 2016;31(4):340-348.
54. Vanova M, Irazoki E, García-Casal JA, et al. The effectiveness of ICT-based neurocognitive and psychosocial rehabilitation programmes in people with mild dementia and mild cognitive impairment using GRADIOR and ehcoBUTLER: study protocol for a randomised controlled trial. Trials. 2018;19(1):100. doi:10.1186/s13063-017-2371-z
55. Petersen RC, Smith GE, Waring SC, Ivnik RJ, Kokmen E, Tangelos EG. Aging, Memory, and Mild Cognitive Impairment. Int Psychogeriatr. 1997;9(S1):65-69. doi:10.1017/S1041610297004717
56. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state.” J Psychiatr Res. 1975;12(3):189-198. doi:10.1016/0022-3956(75)90026-6
57. Rosen WG, Mohs RC, Davis KL. A new rating scale for Alzheimer’s disease. American Journal of Psychiatry. 1984;141(11):1356-1364. doi:10.1176/ajp.141.11.1356
58. Alexopoulos GS, Abrams RC, Young RC, Shamoian CA. Cornell scale for depression in dementia. Biol Psychiatry. 1988;23(3):271-284. doi:10.1016/0006-3223(88)90038-8
59. Ng PEM, Nicholas SO, Wee SL, et al. Implementation and effectiveness of a multi-domain program for older adults at risk of cognitive impairment at neighborhood senior centres. Sci Rep. 2021;11(1):3787. doi:10.1038/s41598-021-83408-5
60. Gambella E, Margaritini A, Benadduci M, et al. An integrated intervention of computerized cognitive training and physical exercise in virtual reality for people with Alzheimer’s disease: The jDome study protocol. Front Neurol. 2022;13. doi:10.3389/fneur.2022.964454
61. Corbett A, Owen A, Hampshire A, et al. The Effect of an Online Cognitive Training Package in Healthy Older Adults: An Online Randomized Controlled Trial. J Am Med Dir Assoc. 2015;16(11):990-997. doi:10.1016/j.jamda.2015.06.014
62. Rai HK, Schneider J, Orrell M. An Individual Cognitive Stimulation Therapy App for People with Dementia and Carers: Results from a Feasibility Randomized Controlled Trial (RCT). Clin Interv Aging. 2021;Volume 16:2079-2094. doi:10.2147/CIA.S323994
63. Cavallo M, Hunter EM, van der Hiele K, Angilletta C. Computerized Structured Cognitive Training in Patients Affected by Early-Stage Alzheimer’s Disease is Feasible and Effective: A Randomized Controlled Study. ARCHIVES OF CLINICAL NEUROPSYCHOLOGY. 2016;31(8):868-876. doi:10.1093/arclin/acw072
64. Beishon L, Evley R, Panerai RB, et al. Effects of brain training on brain blood flow (The Cognition and Flow Study—CogFlowS): protocol for a feasibility randomised controlled trial of cognitive training in dementia. BMJ Open. 2019;9(5):e027817. doi:10.1136/bmjopen-2018-027817
65. Singh MAF, Gates N, Saigal N, et al. The Study of Mental and Resistance Training (SMART) Study—Resistance Training and/or Cognitive Training in Mild Cognitive Impairment: A Randomized, Double-Blind, Double-Sham Controlled Trial. J Am Med Dir Assoc. 2014;15(12):873-880. doi:10.1016/j.jamda.2014.09.010
66. Cavallo M, Angilletta C. Long-Lasting Neuropsychological Effects of a Computerized Cognitive Training in Patients Affected by Early Stage Alzheimer’s Disease: Are They Stable Over Time? Journal of Applied Gerontology. 2019;38(7):1035-1044. doi:10.1177/0733464817750276
67. González-Palau F, Franco M, Bamidis P, et al. The effects of a computer-based cognitive and physical training program in a healthy and mildly cognitive impaired aging sample. Aging Ment Health. 2014;18(7):838-846. doi:10.1080/13607863.2014.899972
68. Vanova M, Irazoki E, García-Casal JA, et al. The effectiveness of ICT-based neurocognitive and psychosocial rehabilitation programmes in people with mild dementia and mild cognitive impairment using GRADIOR and ehcoBUTLER: study protocol for a randomised controlled trial. Trials. 2018;19(1):100. doi:10.1186/s13063-017-2371-z
69. Bonnechère B, Klass M, Langley C, Sahakian BJ. Brain training using cognitive apps can improve cognitive performance and processing speed in older adults. Sci Rep. 2021;11(1):12313. doi:10.1038/s41598-021-91867-z
70. Norris JE, McGeown WJ, Guerrini C, Castronovo J. Aging and the number sense: preserved basic non-symbolic numerical processing and enhanced basic symbolic processing. Front Psychol. 2015;6. doi:10.3389/fpsyg.2015.00999
71. Pertl MT, Benke T, Zamarian L, et al. Do Patients with Mild Cognitive Impairment Understand Numerical Health Information? Journal of Alzheimer’s Disease. 2014;40(3):531-540. doi:10.3233/JAD-131895
72. Leung C, Wong KC, So WWY, et al. The application of technology to improve cognition in older adults: A review and suggestions for future directions. Psych J. 2022;11(4):583-599. doi:10.1002/pchj.565
73. He Z, Dieciuc M, Carr D, et al. New opportunities for the early detection and treatment of cognitive decline: adherence challenges and the promise of smart and person-centered technologies. BMC Digital Health. 2023;1(1):7. doi:10.1186/s44247-023-00008-1
© The Authors 2024