F.G. Wittmann1, A. Pabst1, A. Zülke1, M. Luppa1, I. Blotenberg4, M.I. Cardona4, A. Bauer5, S. Fuchs5, I. Zöllinger6, L. Sanftenberg6, C. Brettschneider7, J. Döhring8, L. Lunden8, D. Czock2, B. Wiese3, J.R. Thyrian4,9,10, W. Hoffmann4,9, T. Frese5, J. Gensichen6, H.-H. König7, H. Kaduszkiewicz8,*, S.G. Riedel-Heller1,*
1. Institute of Social Medicine, Occupational Health and Public Health (ISAP), University of Leipzig, Leipzig, Germany; 2. Department of Clinical Pharmacology and Pharmacoepidemiology, Heidelberg University Hospital, Heidelberg, Germany; 3. Institute for General Practice, Work Group Medical Statistics and IT-Infrastructure, Hannover Medical School, Hannover, Germany; 4. Institute for Community Medicine, University Medicine Greifswald (UMG), Greifswald, Germany; 5. Institute of General Practice and Family Medicine, Martin-Luther-University Halle-Wittenberg, Halle, Saale, Germany; 6. Institute of General Practice/Family Medicine, University Hospital of LMU Munich, Munich, Germany; 7. Department of Health Economics and Health Service Research, University Medical Centre Hamburg-Eppendorf, Hamburg, Germany; 8. Institute of General Practice, University of Kiel, Kiel, Germany; 9. German Centre for Neurodegenerative Diseases (DZNE), site Rostock/ Greifswald, Greifswald, Germany; 10. Faculty V: School of Life Sciences, University of Siegen, Siegen, Germany; * Shared last authorship
Corresponding Author: Felix G. Wittmann, Institute of Social Medicine, Occupational Health and Public Health (ISAP), Medical Faculty, University of Leipzig, Phillip-Rosenthal-Str. 55, 04103 Leipzig, Germany, E-Mail: felix.wittmann@medizin.uni-leipzig.de
J Prev Alz Dis 2024;2(11):348-355
Published online January 15, 2024, http://dx.doi.org/10.14283/jpad.2024.13
Abstract
INTRODUCTION: Differences between women and men matter in the prevalence and risk factors of dementia. We aimed to examine potential sex differences regarding the effectiveness by running a secondary analysis of the AgeWell.de trial, a cluster-randomized multicenter multi-domain lifestyle intervention to reduce cognitive decline.
METHODS: Intention-to-treat analyses of women (n=433) and men (n=386) aged 60 to 77 years were used for models including interactions between intervention group allocation and sex followed by subgroup analysis stratified by sex on primary and secondary outcomes. Further, the same procedure was repeated for age groups (60-69 vs. 70-77) within sex-specific subgroups to assess the effectiveness in different age groups. Trial registration: German Clinical Trials Register (ref. number: DRKS00013555).
RESULTS: No differences were found between women and men in the effectiveness of the intervention on cognitive performance. However, women benefitted from the intervention regarding depressive symptoms while men did not. Health-related quality of life was enhanced for younger intervention participants (60-69 years) in both women and men.
CONCLUSION: The AgeWell.de intervention was able to improve depressive symptoms in women and health-related quality of life in younger participants. Female participants between 60 and 69 years benefited the most. Results support the need of better individually targeted lifestyle interventions for older adults.
Key words: Dementia, prevention, lifestyle, randomized controlled trial, sex differences.
Introduction
To date, more than 55 million people are living with dementia and the tendency is expected to rise to nearly 140 million in the next twenty-five years (1). Currently, available treatments can improve cognitive function but do not provide a cure for cognitive decline (2). Risk reduction interventions are a significant area of interest in dementia research, aligned with the strategic goal 15 of the recent blueprint for dementia research by the World Health Organization (WHO) (3).
While there are non-modifiable risk factors such as aging and the Apolipoprotein E genotype, Livingston and colleagues identified a set of twelve modifiable risk factors (1). Together, those risk factors account for up to 40% of the risk for dementia. Apart from low education and detrimental health behaviors such as smoking, alcohol consumption or physical inactivity, other relevant factors include social isolation, depression, air pollution and health issues like hearing loss, brain injury, hypertension, obesity and diabetes (1).
Sex differences can be observed in the prevalence of dementia, which is higher in women (4). Also, risk factors for cognitive decline differ between men and women in both the severity as well the as the effect on cognition (5-7). Well-known examples of those differences include hypertension, which is more prevalent in men (8), and stress, which is stronger in women (7). Furthermore, certain factors are specific to one sex, such as andropause for men and early menopause for women, respectively (7). Evidence suggests additional age-related differences. Notably, depression and sleep disturbances are stronger risk factors in women at late-life (9).
The last two decades have seen a growing trend towards multi-domain lifestyle interventions targeting cognitive decline. A first approach was the Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER) (10). The study reported small but positive intervention effects on global cognition, domain-specific cognitive function and physical functioning (11). To adjust the approach to different economic, cultural and regional environments, the WW-Fingers network was founded. A trial tailored to the German health care setting was the AgeWell.de-trial. Although no significant effect on global cognition performance was observed in AgeWell.de, the intervention trial demonstrated improvements in health-related quality of life in the overall sample (12).
Evidence indicates sex differences in the risk of dementia and corresponding lifestyle factors, as described above. Moreover, a review by Zülke and colleagues suggests modest benefits of one or two-domain lifestyle interventions on cognition specifically for women (13). Furthermore, sex differences are also observed in previous research for aspects of the intervention. For example are older men found to be more likely to be physical active than older women (14). Women, however, tend to eat healthier than men (15). Regarding social activity, there is a consensus, that social isolation and disengagement are risk factors for cognitive decline16, whereby recent studies suggest higher benefits of social engagement for women (17). Indeed, much less is known, however, about the differences between women and men regarding the effectiveness of multi-domain lifestyle interventions. Results of the FINGER did not find differences between women and men in the effectiveness of the intervention on cognitive outcomes (18).
The objective of this study was to investigate the relationship between sex and the effectiveness of the AgeWell.de intervention. The secondary analyses of the trial followed three research questions: 1) what are the differences between men and women in the effectiveness of the AgeWell.de-intervention on cognitive performance and secondary outcomes at follow-up? 2) How effective was the AgeWell.de intervention on cognitive performance and secondary outcomes at follow up for different age groups within women and men?
Methods
Study design & participants
AgeWell.de was a 2-year multi-domain cluster-randomized intervention trial to preserve cognitive function in older adults at increased risk for dementia. Sample size was calculated based on assumptions of differences in change between groups, statistical power, cluster size and intra-cluster correlation and a dropout rate of ≤ 10% in both groups. Participants were recruited from five study sites across Germany (Leipzig, Greifswald, Halle, Kiel and Munich) by general practitioners (GP). Participating GP practices were used as clusters for randomization, which was carried out by the data management centre at the Institute for General Practice of the Hannover Medical School using a computerized block-randomization algorithm. Study design has been described in detail preciously including statistical power analysis, sample size, recruitment, randomization, intervention procedure and baseline characteristics (19, 20).
The intervention was composed of several components: nutritional counselling, enhancement of physical activity, social activity and cognitive training, management of cardiovascular risk factors, optimization of medications and intervention in bereavement, depressiveness and grief following loss experiences. Intervention components were instructed by trained study nurses during the baseline visit at the participants’ homes. Participants of the control group (CG) received the usual GP treatment and additional written health advice on the intervention components. Randomization and intervention procedure have been particularized in detail previously (12, 20).
The intervention trial was conducted with older adults at risk for dementia with respect to the inclusion criteria of a CAIDE Dementia Risk Score ≥9 (assessed by the GP; (21)) and an age of 60 years and older. Exclusion criteria were diagnosed dementia, severe impairments of hearing, vision or mobility, diseases, which might hinder the implementation of the intervention, insufficient command of the German language or participation in another intervention. Of initially 1,030 participants who completed the baseline assessment, 819 (n=433 women; n=386 men) completed the follow-up assessment 24 months after baseline and were thus used for analysis.
The responsible ethics boards of the coordinating study center of AgeWell.de (Ethics Committee of the Medical Faculty of the University of Leipzig; ethical vote number: 369/17-ek) and of all participating study sites approved the AgeWell.de-study. Participants provided written informed consent to participate at their respective study center. AgeWell.de is registered at the German Clinical Trials Register (DRKS; ID: DRKS00013555).
Outcomes
The main outcome was cognitive performance at follow-up using the neuropsychological test battery mainly based on a subset of the Consortium of Establish a Registry for Alzheimer’s Disease (CERAD) (22) at both baseline and follow-up according to the DSM-V-criteria (23). The test battery consists of measurements of the following cognitive domains: executive function using (Trail Making Test, TMT-B – TMT-A (24)), learning (CERAD Wordlist Memory (22)), language (CERAD Verbal Fluency Test “Animals” (22)), perceptual-motor skills (CERAD Constructional Practice (22)) and social cognition (Reading the Mind in the Eyed test, revised version (25)), which were operationalised by one composite z-score.
Secondary outcomes were mortality, nursing home placement, functioning in activities of daily living, functioning of instrumental activities of daily living, quality of life, health-related quality of life, depressive symptoms, social inclusion and resource utilization and costs. Comparative analyses of nursing home placement (n=1) and mortality (n=11) were not carried out due to insufficient cases; cost-effectiveness will be part of the economic analysis which is still pending. Activities of daily living was compiled by two instruments, the Barthel Index (26) (Activity of Daily Living, ADL) and the Amsterdam-IADL-scale (27) for instrumental activities of daily living. Due to a highly left skewed distribution, the Barthel-Index was dichotomized (100 vs <100). Quality of life was analysed using the World Health Organization Quality of Life questionnaire (WHQOL-OLD) and health-related quality of life (HR-QOL) using the EuroQol visual analogue scale (EQ-VAS; (28)), depressive symptoms using the Geriatric Depression Scale-15 (GDS; (29, 30)) and social inclusion using the Lubben Social Network Scale (LSNS; (31)). Details about the instruments used in AgeWell.de were described previously by Zülke and colleagues (20).
Statistical analysis
Intention-to-treat (ITT) analyses were performed including all participants who completed the follow-up assessment. Examination of incomplete data revealed that missingness was at random (MAR). No evidence of systematic missingness was found using an extra-generated index variable to indicate missingness of information of any variable at baseline. Missing data were therefore imputed by chained equations using multiple imputations (32, 33). All analyses contain pooled estimates of 50 imputed data.
The outcomes were transformed into z-scores by standardizing to the baseline mean and standard deviation, apart from depressive symptoms (used with original scale) and the Barthel-Index (dichotomized). Overall cognitive performance was composed of the z-standardized scores of the six domain specific tests (range -2.711 to 1.394). To have a good trade-off between validity of outcomes and a minimized risk of selection bias, a minimum of three out of the six tests used for the composite score had to be available to calculate the primary outcome.
The models were run using generalized linear regression models (GLM). All outcomes were normally distributed except for activities of daily living (ADL) which was used dichotomized and thus calculated with binomial distribution and depressive symptoms, for which we used GLMs with negative binomial distribution errors. All other models were calculated with identity link and Gaussian distribution errors. The models were all controlled for age (except for the age-stratified models), education (using the Comparative Analysis of Social Mobility in Industrial Nations (CASMIN)-scale (34)), and familial status. Further, all analysis were run using cluster-robust standard errors. Reported results are treatment effects as average marginal effects (AME) with a significance alpha-level set at 0.05 (two-tailed). Data management and analysation were performed using STATA, Version 16 (StataCorp, College Station, TX, USA).
In a first step, we ran GLMs with interaction terms between the indicators of the research questions (1) sex, (2) age-group within women and (3) age-group within men (all interacting with the intervention group (IG)) to assess whether a potential intervention effect varied by subgroup on each outcome, respectively. Second, we performed GLMs on the outcomes for women and men after stratifying the sample into female and male subsamples. For the analysis within women and men, were further stratified the male and female samples by age (60-69 and 70-77 years old). In addition, two-way interaction analyses were carried out with each subgroup indicator and treatment group. The results of the interaction analysis and the subgroup analyses are depicted together for better interpretability (tables 2 & 3).
Results
Characteristics of male and female participants
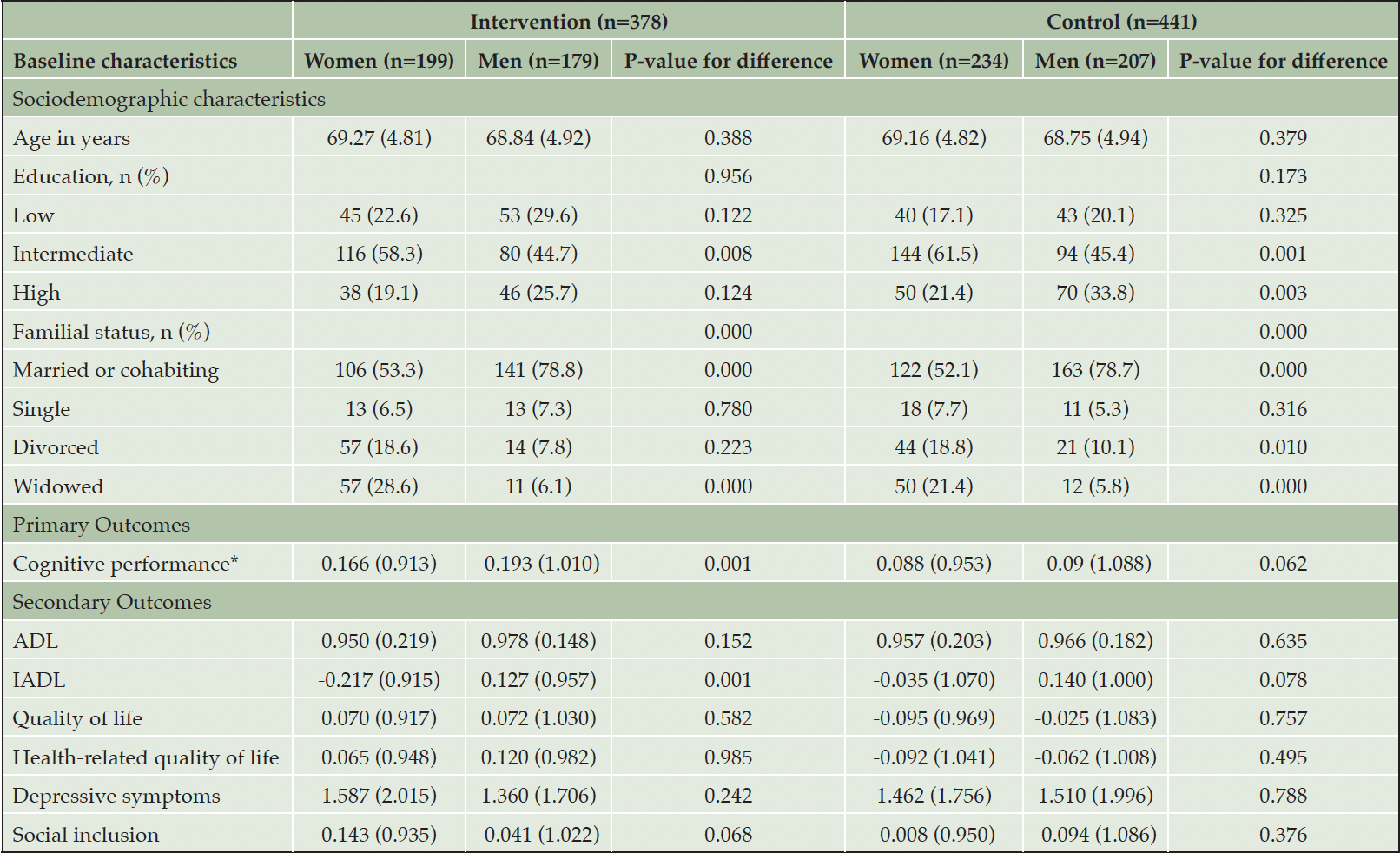
Participant characteristics are summarized in Table 1. Of the 819 participants who completed the follow-up assessments, 52.9% (n=433; IG: n=199; CG: n=234) were female and 47.1% (n=386; IG: n=179; CG: n=207) were male; the participants age ranged between 60 and 77 years. Differences between women and men were observed for both groups for intermediate education that was bigger in women, whereby the proportion of participants with a high level of education was bigger in men. In addition, differences were found in the family status. More women than men were widowed, while more men than women were married or cohabitating. Only a very small proportion of male participants was widowed or single. Differences in the outcomes were observed for cognitive performance, which was higher for female participants and only in the intervention group, as well as instrumental activities of daily living, which was higher in men for the intervention recipients.
Note. Data are mean (SD) or n(%); *cognitive performance = composite score; Primary and secondary outcomes are z-scores (standardized to BL mean and SD; higher values indicate better performance) except of perceptual-motor abilities, depressive symptoms (original score) and ADL (dichotomized score); ADL: Activities of daily Living; BL: baseline; IADL: Instrumental Activities of Daily Living; SD: standard deviation.
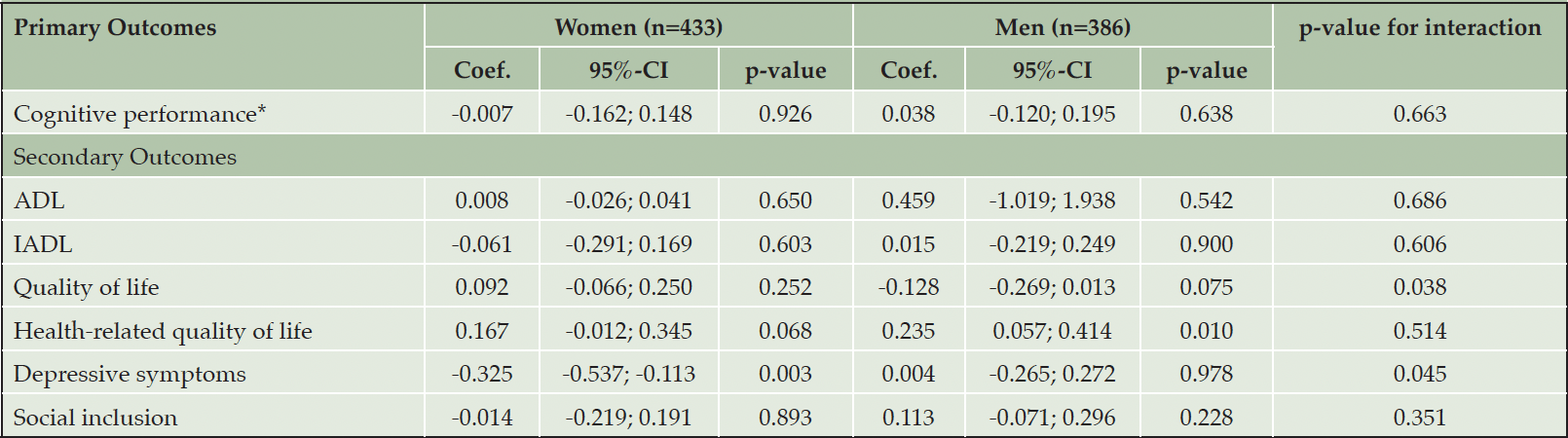
Table 2. Between-group differences in primary and secondary outcomes at follow-up, stratified by sex; including results of GLMs with interaction-term of intervention group×sex on each outcome
Note. The models are adjusted for age (continuous), education, family status and baseline score of the respective outcome. GLMs were used for between-group differences (IG vs. CG) at follow-up for the sex-stratified subsamples; models with interaction (reference group: women) were calculated for the sample as a whole; coefficients are estimated mean group differences (IG vs. CG) in scores at follow-up. Outcomes are z-scores except of ADL (dichotomized) and depressive symptoms (original scores); ADL: Activities of Daily Living; IADL: Instrumental Activities of Daily Living; CI: confidence interval; *composite score
Differences in the effectiveness between men and women
No differences were found in the effectiveness for cognitive performance between women and men (ßwomen=0.009, p=0.892; ßmen=0.037, p=0.590); accordingly, no effect was found for the interaction of treatment group and sex (p=0.663). Regarding secondary outcomes, significant interaction terms for sex and intervention on quality of life (p=0.038) and depressive symptoms (p=0.045) indicate differences between women and men. The results of the additional models reveal, nevertheless, that there was no further difference in the effectiveness of the intervention on quality of life between women and men. However, we found a difference in the effectiveness between women and men for depressive symptoms. While women benefitted from the intervention respective depressive symptoms (ß=-0.325; p=0.003), no effect was found for men (ß=0.004; p=0.978). In addition, a positive intervention effect on health-related quality of life was found for men (ß=0.057; p=0.010), which, however, was not significantly different from women related to the p-value of the interaction (p=0.514).
Differences in the effectiveness between age groups in women
The results of the age-stratified analysis of the female group are set out in Table 3. Women between 60-69 years benefitted from the intervention in their perceived health-related quality of life (ß=0.434, p<0.001), whereas women 70 years and older did not (ß=-0.095, p=0.513; p-value of interaction=0.005). We did not find further differences between the age groups for women. Strong effectiveness of the intervention for women in reducing depressive symptoms was also found in both age-stratified groups, whereby the effect of the intervention on depressive symptoms was slightly stronger for women aged 70 years and older (60-69 years: ß=0.343; p=0.017 vs. 70-77 years: ß=0.352; p=0.020). Furthermore, no statistical significance of the interaction term supports the result that there are no further differences between age groups for the effectiveness of the intervention on depressive symptoms in women.
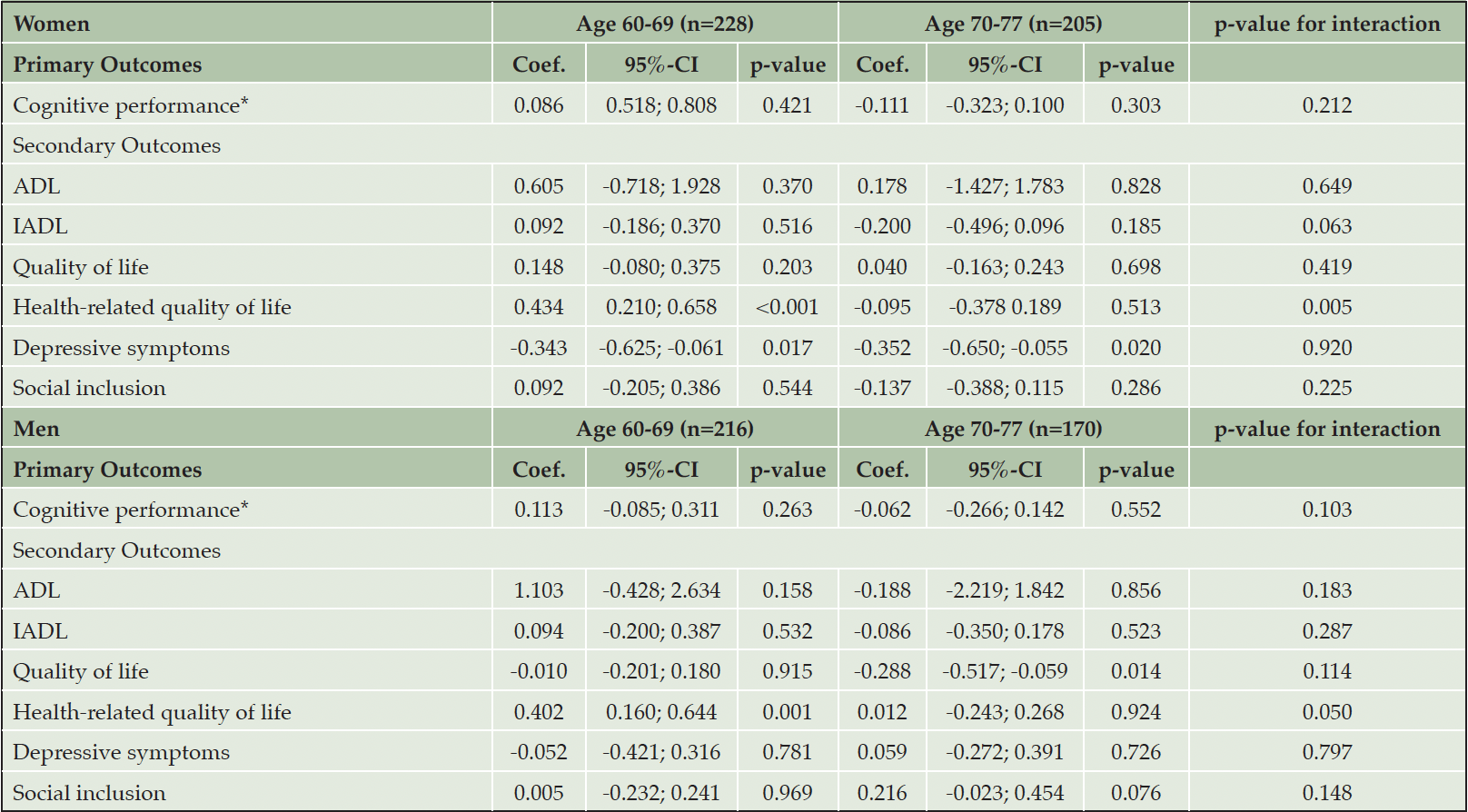
Table 3. Between-group differences in primary and secondary outcomes at follow-up, stratified by age for women and men; including results of GLMs with interaction-term of intervention group×age for the sex-stratified models on each outcome
Note. The models are adjusted for age (continuous), education, family status and baseline score of the respective outcome. GLMs were used for between-group differences (IG vs. CG) at follow-up for the sex-stratified subsamples; models with interaction were calculated for the sample as a whole; coefficients are estimated mean group differences (IG vs. CG) in scores at follow-up. Outcomes are z-scores except of ADL (dichotomized) and depressive symptoms (original scores); ADL: Activities of Daily Living; IADL: Instrumental Activities of Daily Living; CI: confidence interval; *composite score
Differences in the effectiveness between age groups in men
No significant differences of the intervention were found between the age groups for the male group on cognitive performance. Respective secondary outcomes, we found a negative effect for quality of life for men between 70 and 77 (ß=-0.288; p=0.014). However, no effect was found for men between 60-69 for quality of live (-0.010, p=0.915). Instead, men between 60-69 years reported significantly better health-related quality of life when participating in the intervention (ß=0.402, p= 0.001) compared with men who were 70 years and older (ß=0.012, p=0.924; p-value of interaction=0.05). The significant interaction term for health-related quality of life for men strongly supports the assumption of a difference (p=0.050).
Discussion
The objective of this study was to examine differences in the effectiveness of the AgeWell.de -intervention between women and men, as well as within each sex group.
The primary outcome of the AgeWell.de-trial was global cognitive performance. No significant effects were observed for either men or women, or within the subgroups. Hence, no differences between the groups were found for cognitive performance. However, a difference between women and men was found regarding depressive symptoms. The intervention had a positive effect for female intervention group participants but not for men. Generally, participants in the younger age group (60-69) benefited in terms of better health-related quality of life in both men and women.
While several lifestyle interventions against cognitive decline based on the FINGER have been implemented10, to our knowledge, there is currently limited evidence regarding sex differences in the effectiveness of multi-domain lifestyle interventions so far. Consistent with the findings of the FINGER-multidomain intervention, our observations also indicate no sex differences in the effectiveness on cognitive performance (18).
However, we identified variations in the effectiveness on depressive symptoms. It must be taken into account, that the level of depressiveness was moderate at the beginning of the intervention. Nevertheless, evidence on sex differences in depression among older adults reports higher scores in women (35) while evidence offers mixed findings about sex differences in response to treatments of depressiveness (36). Main reasons for depressive symptoms among adults who are 60 and older are widowhood or living alone, poor health or poverty (35). It seems possible that this result is due to a better-targeted intervention for women in terms of depressive symptoms. First, even if no differences between depressive symptoms were found between men and women at baseline, women and men strongly differed in being widowed which is seen as risk factor. The analyses were adjusted for family status but it would be of interest if having a partner or living alone had an influence on the adherence and effectiveness of the intervention. Also, two of the intervention components were composed of the enhancement of social activity, which is seen as directly linked to reduce depressive symptoms (37, 38), and secondly, individual intervention for bereavement, grief & depressive symptoms. Analyses towards the intervention components and respective adherence might reveal further insights about the reasons, why women benefited more than men did regarding depressive symptoms. In fact, we are actually analysing the effect on depressiveness and the association between the change and the components of the intervention in greater depth which is to be reported in a more detailed manuscript. However, the result is gratifying, since depressiveness as risk factor is stronger in women at late-life (9).
No sex difference was found regarding intervention effects on health-related quality of life. Interestingly, we found differences between younger and older intervention participants in both women and men. This is in line with the main results of the AgeWell.de-trial, where a positive effect was found for the overall sample with a negative interaction with continuous age (12). Surprisingly, we found a negative effect on quality of life in men between 70-77 years. Together with the results on health-related quality of life, this might suggest, that treatments are more efficient in younger participants. A qualitative study about aspects of lifestyle change in older adults reported that retirement was seen as auspicious moment to implement lifestyle changes – which would support the assumption that younger persons benefitted more than older ones (39).
The need of more targeted interventions is clearly supported by the current findings. Differences in the effectiveness between both men and women and further different ages reveal those needs. Considerably more work will be needed to determine the differences between women and men, for example in the qualitative analysis of adherence to lifestyle interventions. Qualitative studies could further be a promising approach to analyse the tangible differences between women and men in the effectiveness but also the adherence of interventions. Besides, meta-analysis of several multidomain randomized clinical trials as quantitative approach could be worthwhile. Another strategy might be the inclusion of potential participants in designing future intervention studies to enhance effectiveness.
Limitations
This study has several limitations, some of which were bound to the intervention itself. As discussed in the main trial paper, a limitation is the trial duration, which might be too short for significant effects on cognitive performance (12). Furthermore, the control group received GP treatment as usual. Different check-ups for pre-existing diseases or certain aspects of prevention are usually covered in GP treatment, so that this has as a quite high standard in Germany. At last, AgeWell.de was completed during the COVID-19 pandemic, whereby perceived restrictions were more frequently reported for participants of the intervention, especially for nutrition (12).
Conclusion
This study has identified beneficial effects of the AgeWell.de intervention on depressive symptoms in women and on health-related quality of life in women and men between 60 and 69 years. Concluding, this study supports evidence of stronger effects of lifestyle interventions for women between 60 and 69 years. Thus, this study supports the need of intervention designs that are closer tailored for specific subgroups.
Acknowledgements: Members of the AgeWell.de-study group: Principal Investigator: Steffi G. Riedel-Heller; Co-Principal Investigators: Wolfgang Hoffmann, Jochen Gensichen, Walter E. Haefeli, Hanna Kaduszkiewicz, Hans-Helmut König, Thomas Frese, David Czock, Jochen René Thyrian; Franziska Berg, Andrea Bischhoff, Christian Brettschneider; Mandy Claus, Juliane Döhring, Alexander Eßer, Corinna Gräble, Stephanie Hingst, Caroline Jung-Sievers, Kerstin Klauer-Tiedtke, Kerstin Krebs-Hein, Flora Wendel, Sebastian Lange, Paula Liegert, Dagmar Lochmann, Tobias Luck, Melanie Luppa, Silke Mamone, Lea Markgraf, Andreas Meid, Michael Metzner, Lydia Neubert, Anke Oey, Susanne Röhr, Franziska-Antonia Zora Samos, Karin Schumacher, Theresa Terstegen, Anne Henrike Wagner, Lars Wamsiedler, Tanja Wehran, Marina Weißenborn, Ines Winkler, Isabel Zöllinger, Andrea Zülke, Ina Zwingmann. The authors want to thank all participating GP practices and study participants of the AgeWell.de-trial. We acknowledge support from Leipzig University within the program of Open Access Publishing.
Conflict of Interest: None declared.
Authors Contributions: Conceptualization of the AgeWell.de-trial: D.C., J.G., W.E.H., W.H., H.K., H.-H.K., J.R.T., B.W. and S.G.R.-H.; Analysis and Interpretation of Data and Writing the Original Draft: F.W.; Funding Acquisition: D.C., J.G., W.E.H., W.H., H.K., H.-H.K., J.R.T., B.W., S.R.-H.; Reviewing and Editing: A.P., A.Z., M.L., I.B., M.I.C., A.B., S.F., I.Z., L.S., C.B., J.D., L.L., D.C., B.W., J.R.T., W.H., T.F., J.G., H.-H.K., H.K., S.R.-H.. All authors contributed to the article and approved the submitted version.
Funding: Open Access funding enabled and organized by the Open Access Publishing Fund of Leipzig University supported by the German Research Foundation within the program Open Access Publication Funding. This publication is part of the study “AgeWell.de – a multi-centric cluster-randomized controlled prevention trial in primary care” and was funded by the German Federal Ministry for Education and Research (BMBF; grants: 01GL1704A, 01GL1704B, 01GL1704C, 01GL1704D, 01GL1704E, 01GL1704F). The BMBF had no role in the design of this study and has no role during its execution, analyses, interpretation of the data, or decision to submit results. Open Access funding enabled and organized by Projekt DEAL
Ethical standards: AgeWell.de was reviewed and approved by the Ethical Committee at the Medical Faculty, Leipzig University; Ethical Committee at the Medical Faculty, Christian-Albrechts-University, Kiel; Ethical Committee at Greifswald University Medicine; Ethical Committee at the Medical Faculty, Ludwig-Maximilian-University, Munich; Ethical Committee at the Medical Faculty, Martin-Luther-University Halle-Wittenberg; Ruprecht-Karls-University, Heidelberg. The participants provided their written informed consent to participate in this study.
Open Access: This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
References
1. Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. The Lancet. 2020;396(10248):413-446. https://doi.org/10.1016/S0140-6736(20)30367-6.
2. Yiannopoulou KG, Papageorgiou SG. Current and future treatments in Alzheimer disease: an update. Journal of central nervous system disease. 2020;12:1179573520907397. https://doi.org/10.1177/117957352090739.
3. Dua T, Kestel D, Swaminathan S, et al. A blueprint for dementia research. Geneva; 2022.
4. Cao Q, Tan C-C, Xu W, et al. The prevalence of dementia: a systematic review and meta-analysis. Journal of Alzheimer’s Disease. 2020;73(3):1157-1166. https://doi.org/10.3233/jad-191092.
5. Kim M-Y, Kim K, Hong CH, Lee SY, Jung Y-S. Sex differences in cardiovascular risk factors for dementia. Biomolecules & therapeutics. 2018;26(6):521. https://doi.org/10.4062%2Fbiomolther.2018.159.
6. Ferretti MT, Martinkova J, Biskup E, et al. Sex and gender differences in Alzheimer’s disease: current challenges and implications for clinical practice: Position paper of the Dementia and Cognitive Disorders Panel of the European Academy of Neurology. European journal of neurology. 2020;27(6):928-943. https://doi.org/10.1111/ene.14174.
7. Sindi S, Toopchiani S, Barbera M, et al. Sex and gender differences in genetic and lifestyle risk and protective factors for dementia. In: Sex and Gender Differences in Alzheimer’s Disease. Elsevier; 2021:269-308.
8. Sandberg K, Ji H. Sex differences in primary hypertension. Biology of sex differences. 2012;3(1):1-21. https://doi.org/10.1186/2042-6410-3-7.
9. Ewelina Biskup, Valeria Jordan, Beatrice Nasta, Katrin Rauen. Chapter 7 – Sex differences in psychiatric disorders and their implication for dementia. In: Maria Teresa Ferretti, Annemarie Schumacher Dimech, Antonella Santuccione Chadha, eds. Sex and Gender Differences in Alzheimer’s Disease. Academic Press; 2021:187-206.
10. Kivipelto M, Mangialasche F, Snyder HM, et al. World-Wide FINGERS Network: a global approach to risk reduction and prevention of dementia. Alzheimer’s & Dementia. 2020;16(7):1078-1094. https://doi.org/10.1002/alz.12123.
11. Ngandu T, Lehtisalo J, Solomon A, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. The Lancet. 2015;385(9984):2255-2263. https://doi.org/10.1016/S0140-6736(15)60461-5.
12. Zülke AE, Pabst A, Luppa M, et al. A multidomain intervention against cognitive decline in an at-risk-population in Germany – Results from the cluster-randomized AgeWell.de-trial. Alzheimer’s & Dementia (Under final review). 2023.
13. Zülke AE, Riedel-Heller SG, Wittmann F, Pabst A, Röhr S, Luppa M. Gender-Specific Design and Effectiveness of Non-Pharmacological Interventions against Cognitive Decline—Systematic Review and Meta-Analysis of Randomized Controlled Trials. The Journal of Prevention of Alzheimer’s Disease. 2023;10(1):69-82. https://doi.org/10.14283/jpad.2022.80.
14. Sun F, Norman IJ, While AE. Physical activity in older people: a systematic review. BMC public health. 2013;13(1):1-17.
15. Anna H Baker, Jane Wardle. Sex differences in fruit and vegetable intake in older adults. Appetite. 2003;40(3):269-275. doi:10.1016/S0195-6663(03)00014-X.
16. Kelly ME, Duff H, Kelly S, et al. The impact of social activities, social networks, social support and social relationships on the cognitive functioning of healthy older adults: a systematic review. Systematic reviews. 2017;6(1):1-18.
17. Zunzunegui M-V, Alvarado BE, Del Ser T, Otero A. Social Networks, Social Integration, and Social Engagement Determine Cognitive Decline in Community-Dwelling Spanish Older Adults. The Journals of Gerontology: Series B. 2003;58(2):S93-S100. doi:10.1093/geronb/58.2.S93.
18. Sindi S, Kåreholt I, Ngandu T, et al. Sex differences in dementia and response to a lifestyle intervention: Evidence from Nordic population-based studies and a prevention trial. Alzheimer’s & Dementia. 2021;17(7):1166-1178. https://doi.org/10.1002/alz.12279.
19. Röhr S, Zülke A, Luppa M, et al. Recruitment and baseline characteristics of participants in the AgeWell. de Study—A Pragmatic cluster-randomized controlled lifestyle trial against cognitive decline. International journal of environmental research and public health. 2021;18(2):408. https://doi.org/10.3390/ijerph18020408.
20. Zülke A, Luck T, Pabst A, et al. AgeWell. de–study protocol of a pragmatic multi-center cluster-randomized controlled prevention trial against cognitive decline in older primary care patients. BMC geriatrics. 2019;19(1):1-14. https://doi.org/10.1186/s12877-019-1212-1.
21. Kivipelto M, Ngandu T, Laatikainen T, Winblad B, Soininen H, Tuomilehto J. Risk score for the prediction of dementia risk in 20 years among middle aged people: a longitudinal, population-based study. The Lancet Neurology. 2006;5(9):735-741. https://doi.org/10.1016/S1474-4422(06)70537-3.
22. Morris JC, Heyman A, Mohs RC, et al. The consortium to establish a registry for Alzheimer’s disease (CERAD): I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology. 1989. https://doi.org/10.1212/wnl.39.9.1159.
23. American Psychiatric Association. Diagnostic and statistical manual of mental disorders (5th ed.).
24. Reitan RM. Trail Making Test: Manual for administration and scoring. Reitan Neuropsychology Laboratory; 1986.
25. Baron-Cohen S, Wheelwright S, Hill J, Raste Y, Plumb I. The “Reading the Mind in the Eyes” Test revised version: a study with normal adults, and adults with Asperger syndrome or high-functioning autism. The Journal of Child Psychology and Psychiatry and Allied Disciplines. 2001;42(2):241-251. https://doi.org/10.1017/S0021963001006643.
26. Mahoney FI, Barthel DW. Functional evaluation: the Barthel Index: a simple index of independence useful in scoring improvement in the rehabilitation of the chronically ill. Maryland state medical journal. 1965.
27. Sikkes S am, Knol DL, Pijnenburg YAL, Lange-de Klerk ESM de, Uitdehaag BMJ, Scheltens P. Validation of the Amsterdam IADL Questionnaire©, a new tool to measure instrumental activities of daily living in dementia. Neuroepidemiology. 2013;41(1):35-41. https://doi.org/10.1159/000346277.
28. The EuroQol Group. EuroQol-a new facility for the measurement of health-related quality of life. Health policy. 1990;16(3):199-208. https://doi.org/10.1016/0168-8510(90)90421-9.
29. Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. Clinical Gerontologist: The Journal of Aging and Mental Health. 1986. https://doi.org/10.1300/J018v05n01_09.
30. Gauggel S, Birkner B. Validität und Reliabilität einer deutschen Version der Geriatrischen Depressionsskala (GDS). Zeitschrift für klinische Psychologie. 1999. https://psycnet.apa.org/doi/10.1026/0084-5345.28.1.18.
31. Lubben J, Blozik E, Gillmann G, et al. Performance of an abbreviated version of the Lubben Social Network Scale among three European community-dwelling older adult populations. The Gerontologist. 2006;46(4):503-513. https://doi.org/10.1093/geront/46.4.503.
32. van Buuren S. Flexible imputation of missing data. CRC press; 2018.
33. Sterne JAC, White IR, Carlin JB, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. Bmj. 2009;338. https://doi.org/10.1136/bmj.b2393.
34. Brauns H, Scherer S, Steinmann S. The CASMIN educational classification in international comparative research. https://doi.org/10.1007/978-1-4419-9186-7_11.
35. Girgus JS, Yang K, Ferri CV. The gender difference in depression: are elderly women at greater risk for depression than elderly men? Geriatrics. 2017;2(4):35. https://doi.org/10.3390/geriatrics2040035.
36. Parker G, Blanch B, Crawford J. Does gender influence response to differing psychotherapies by those with unipolar depression? Journal of affective disorders. 2011;130(1-2):17-20. https://doi.org/10.1016/j.jad.2010.05.020.
37. Isaac V, Stewart R, Artero S, Ancelin M-L, Ritchie K. Social activity and improvement in depressive symptoms in older people: a prospective community cohort study. The American Journal of Geriatric Psychiatry. 2009;17(8):688-696. https://doi.org/10.1097/JGP.0b013e3181a88441.
38. Nagy E, Moore S. Social interventions: An effective approach to reduce adult depression? Journal of affective disorders. 2017;218:131-152. https://doi.org/10.1016/j.jad.2017.04.043.
39. Wittmann FG, Zülke A, Schultz A, et al. Beneficial and Impeding Factors for the Implementation of Health-Promoting Lifestyle Interventions—A Gender-Specific Focus Group Study. International journal of environmental research and public health. 2023;20(4):3520. https://doi.org/10.3390/ijerph20043520.
© The Authors 2024