M. Tahmi1, J.A. Luchsinger2
1. Department of Neurology, State University of New York Downstate Medical Center, new York, USA; 2. Departments of Medicine and Epidemiology, Columbia University Irving Medical Center, New York, USA
Corresponding Author: José A. Luchsinger, MD MPH; Columbia University Irving Medical Center, 622 West 168th Street, PH9 Center room 210, New York, NY 10032; Tel: 212-305-4730; Fax: 212-305-9349; Email: jal94@cumc.columbia.edu
J Prev Alz Dis 2023;4(10):706-717
Published online October 24, 2023, http://dx.doi.org/10.14283/jpad.2023.113
Abstract
Metformin is a safe and effective medication for Type 2 diabetes (T2D) that has been proposed to decrease the risk of aging related disorders including Alzheimer’s Disease (AD) and AD related disorders (ADRD). This review seeks to summarize findings from human and non-human studies examining the association of metformin with AD/ADRD related outcomes. Studies in animal models suggest that metformin could decrease the risk of AD/ADRD through multiple mechanisms including neuroprotective effects, decreasing neuroinflammation, and decreasing AD pathology. However, there are non-human studies that suggest that metformin could increase the risk of AD/ADRD. Observational human studies are also conflicting, but those with better study designs suggest that metformin use in persons with T2D is associated with a lower risk of dementia. However, these observational studies are limited by the use of administrative data to ascertain metformin use and/or cognitive outcomes. There are few clinical trials in persons without T2D that have small sample sizes and short durations but suggest that metformin could prevent AD/ADRD. There are ongoing studies including large clinical trials with long duration that are testing the effect of metformin on AD/ADRD outcomes in persons without T2D at risk for dementia.
Key words: Metformin, diabetes, dementia, Alzheimer.
Introduction
Repurposing medications for type 2 diabetes (T2D) for the prevention and treatment of Alzheimer’s disease (AD) and AD related dementias (ADRD) is based broadly on the observation that T2D is related to an increased risk of cognitive impairment including AD and ADRD (1, 2). In addition, there are shared mechanisms between T2D and AD/ADRD (3). Processes that contribute to cognitive impairment and dementia such as cerebrovascular disease (CVD) (4), neuroinflammation (5), oxidative stress (6), defective synapses and cell ageing (7) are present in both AD/ADRD and T2D (8-10). However, it is still unclear whether T2D causes AD, characterized by brain accumulation of plaques of amyloid beta (Aβ) and tau neurofibrillary tangles (11). Most neuropathology studies found no link between T2D and post-mortem AD neuropathology (12-18). Similarly, most in vivo AD biomarker studies reported no association between T2D and brain Aβ ascertained by Positron Emission Tomography (PET) (19-21), or cerebrospinal fluid (CSF) (20, 22). In most of these studies T2D was related to biomarkers of neurodegeneration ascertained on Magnetic Resonance Imaging (MRI) (20), CSF t-tau (20) or fluorodeoxyglucose (FDG) PET (21). Fewer studies have reported an association between T2D and higher AD pathology ascertained on post-mortem neuropathology (23), CSF (24) or plasma biomarkers (25). Other studies have reported lower AD pathology among persons with T2D, as ascertained on post-mortem neuropathology (26, 27), Aβ PET imaging (28) and plasma biomarkers (29).
The goal of T2D treatment is to maintain glycemic control, usually measured as hemoglobin A1c (HbA1c), in an ideal range (30). Insulin, an anabolic hormone produced by the pancreas, maintains normoglycemia. A combination of increased insulin demand mediated by insulin resistance and relatively decreased insulin secretion results first in modest hyperglycemia, known as prediabetes or impaired glucose tolerance, which often progresses to T2D (31). Treatment of T2D usually includes increasing insulin levels by administering exogenous insulin or increasing pancreatic insulin secretion, improving insulin sensitivity in peripheral tissues such as the liver and muscle, or a combination of both (30). Newer treatments such as sodium-glucose cotransporter-2 inhibitors (SGLT2i) reduce blood glucose levels by increasing glycosuria (32). While there are 12 classes of T2D medications currently used for treatment of T2D according to the American Diabetes Association treatment guidelines (33), metformin, an insulin sensitizer, is the most commonly used drug world-wide. It is recommended as the first medication to treat T2D with additional medications added, as needed, to maintain target glycemia (33). Metformin has a favorable safety profile and is associated with weight loss (34). Exogenous insulin and the sulfonylureas increase insulin levels and may cause weight gain which can further exacerbate insulin resistance. Insulin and sulfonylurea therapy are also associated with hypoglycemia, which may be related to cognitive impairment (35). Thiazolidinediones are potent insulin sensitizers but are associated with edema and other adverse outcomes (36). Other T2D medications, such as the dipeptidyl peptidase-4 inhibitors (DPP4i), and glucagon-like peptide-1 receptor agonists (GLPR1a), increase insulin secretion, but have other effects that make them weight neutral or lead to weight loss (37, 38) and improved insulin sensitivity. Finally, the SGLT2i are associated with modest weight loss and a reduction in risk for cardiovascular disease (32). While the primary mechanism of action of T2D medications may be either an improvement in insulin secretion or reduced insulin resistance (increased tissue sensitivity), many glucose-lowering medications have secondary effects that encompass both mechanisms. The overarching hypothesis linking T2D medications to AD is that improving peripheral insulin sensitivity affects AD risk through improvements in brain insulin signaling, amyloid clearance, inflammation, and oxidation (39). Thus, the main T2D medications hypothesized to improve cognitive outcomes are those that improve insulin sensitivity including thiazolidinediones, metformin, DPP4i, SGLT2i, and GLP-1ra. Metformin is the most widely used medication in general medical practice, alone or in combination with other T2D medications, as it is first line treatment for T2D (30). It is inexpensive and safe when used in persons without contraindications (advanced renal disease [glomerular filtration rate < 30 ml/min], liver disease other than non-alcoholic fatty liver disease, class III-IV congestive heart failure, alcohol abuse). The most common side effects are related to gastrointestinal intolerance (bloating, diarrhea). Rare side effects include cobalamin deficiency, which may be related to cognitive impairment, and lactic acidosis. In recent years there has been a call for repurposing metformin as a preventive therapy against cognitive decline given it potential beneficial pleotropic effects on conditions other than T2D and obesity including liver disease, cardiovascular disease, cancer, renal disease, and aging in general (Figure 1) (40). Thus, this review focuses on metformin given its potential effects on AD and ADRD.
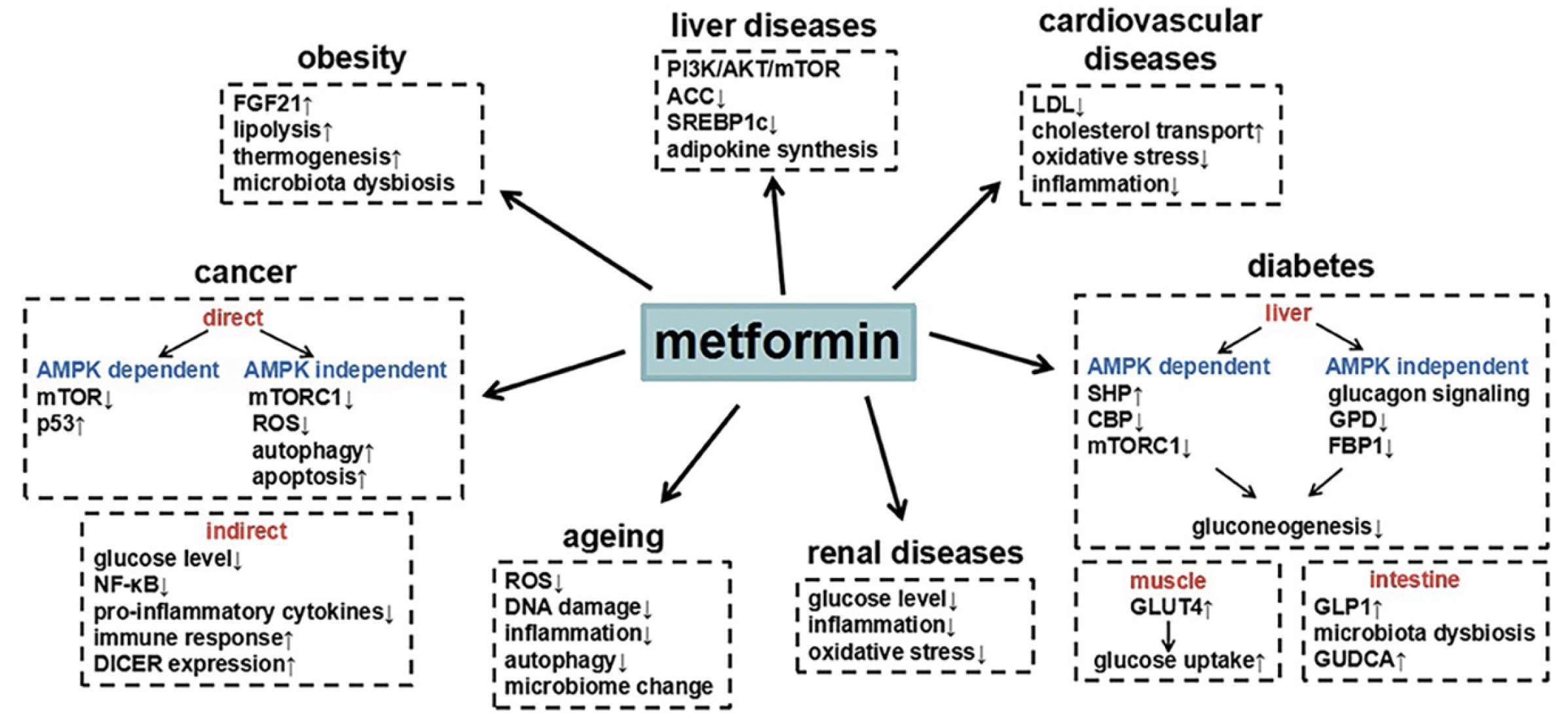
Figure 1. Summary of the effects of metformin on mechanistic pathways related to various disease and conditions
Figure from Lv et al (40)
Methods
This is a narrative review of non-human and human studies, including observational studies and clinical trials, examining the association of metformin with cognitive and brain outcomes. We used PubMed as the main database for our literature search with a focus on English language non-human and human studies including observational studies and clinical trials. We prioritized studies published in the last 10 years from 2013 until June 16, 2023. We searched both original and relevant review articles. We used the search term metformin paired with, cognition, cognitive, dementia, amyloid, tau, Alzheimer’s, neurodegenerative, neurodegeneration in abstract/title of each article or as a Medical Subject Headings (MeSH) term. Reference lists of included studies and relevant review articles were further reviewed to identify additional studies not identified through the pubmed search.
Summary of findings
Metformin in non-human studies
Chen et al first reported the potential for metformin to increase amyloid production in a cell model of neurons in 2009 (41). Since that report many studies have examined the effect of metformin on cognition and neuropathology in animals, which we summarize below.
Non-human studies showing adverse or neutral effects of metformin
In an AD mouse model (AβPP mice), Ditacchio et al (42) reported worse memory function related to oral metformin administration (350 mg/kg/day) in male mice only, while female mice showed enhanced memory. Both opposite sex-dependent effects of metformin were mediated by an activation of the AMPK pathway (42). In a mouse model of diabetes (db/db mice, 18 weeks of intraperitoneal injection of 200mg/kg) (43), metformin decreased hippocampal total and phosphorylated tau, and attenuated the decrease of synaptophysin, an important regulator of synaptic function. However, despite all these neuroprotective effects, metformin had no effect on spatial learning and memory (43). In a study of male Wistar rats, McNeilly and colleagues reported that high-fat diet induced-cognitive impairment was not prevented by the administration of metformin, despite its attenuating actions on insulin resistance and weight gain (44). Thangthaeng et al reported neutral effects of metformin on glucose levels and body weight as well as neutral effects on redox homeostasis, and age-related cognitive, psychomotor, and sensory decline, in a study of young, middle aged and old male mice (C57BL/6) (45). However, impaired spatial memory and visual acuity and reduced antioxidant enzyme (superoxidase dismutase) in the cortex and hippocampus were observed in the metformin treated old mice group (45).
Other animal studies reported deleterious effects of metformin on brain pathology but have not investigated if these effects would have impacted cognitive function (41, 46, 47). Administration of metformin (oral 2-5mg/ml for 06 days) to wild type mice (C57/BL6) resulted in an increase in both intra and extracellular Aβ mediated by an upregulation of β-Site Amyloid Precursor Protein Cleaving Enzyme 1 (BACE1), the enzyme that cleaves Amyloid Precursor Protein (APP) to Aβ peptides. Similar findings were observed when 2 mg of metformin was administered to triple transgenic (3xTg) mouse model of AD (3xTg-AD harboring Presenilin 1 (PS1-M146V), APP (Swe), and tau (P301L)) for 3 months (41). These results were also confirmed on cell cultures of primary cortical neurons and N2a neuroblastoma cells (a mouse neuroblastoma cell line) that express human APP, a cellular model of AD. However, the combination of metformin with insulin resulted in reduced Aβ peptide levels. Similar findings were reported by Picone and colleagues (46) where metformin increased APP and presenilin levels via activation of the Nuclear Factor Kappa-Light-Chain-Enhancer of Activated B Cells (NF-κB) transcription factor resulting in an accumulation of Aβ peptides as well as cellular death mediated by oxidative stress and mitochondrial damage. These effects were confirmed both in vitro (using mice neuroblastoma LAN5 cell lines), in vivo (wild type C57B6 mice for 07 days) and in ex-vivo (metformin added to Human Peripheral Blood Mononuclear Cells derived from young subjects reduced cell variability implying an increase in oxidative stress) and reversed by the addition of insulin (46). Finally, In a study of a mouse model of tauopathy (P301S), Barini et al (48), reported that metformin had paradoxical effects on AD pathology with both increased formation of aggregates with β-sheet and decreased tau phosphorylation in the cortex and the hippocampus resulting in an enhanced abnormal hyperactivity behavior (48).
Non-human studies showing benefits of metformin
In a model of memory impairment, metformin administration for 4 weeks prevented memory impairment induced by L-methionine in adult male Wistar rats, possibly by reducing markers of oxidative stress in the hippocampus (49). Similar findings were reported by Zhao et al (50) who showed that metformin decreased markers of oxidative stress and improved cognitive impairment in pentylenetetrazole-induced kindling mice (50). Far et al (51) reported that metformin improved spatial learning and memory in Senescence Accelerated Prone Mouse (SAMP8), a mouse model of AD, by lowering the c99 fragment of Amyloid Precursor Protein (APPc99) and phosphorylated tau. In a different study (52) Chen and colleagues, found that metformin administration (6 weeks of oral 200 mg/kg) decreased Aβ influx through the Blood Brain Barrier (BBB), decreased hippocampal Aβ40 and Aβ42, inhibited neuronal apoptosis, and improved memory impairment in a diabetes mouse model (db/db). In a different study, the effects of metformin on aged mice were APOE -dependent as reported in a study by Zhang et al, where metformin administration in ApoE3-TR mice showed improved spatial memory. Specifically, metformin inhibited AMPK and activated Mammalian Target of Rapamycin (mTOR) and increased insulin signaling and post-synaptic proteins in the ApoE3-TR mice (53). These effects, however, were not observed in aged ApoE4-TR mice. Surprisingly, metformin also increased tau phosphorylation in old ApoE-TR mice (both E3 and E4) by stimulating Glycogen Synthase Kinase-3β (GSK-3β) in the same study (53). This latter finding seems counterintuitive given the beneficial effects of metformin on cognition reported in the same study and may imply, as the authors concluded, possible side effects of chronic metformin use on brain pathology (53). In another study, metformin administration to a mouse model of AD (APP/PS1 mice) improved their spatial memory. On a molecular level, metformin enhanced hippocampal neurogenesis and decreased Aβ and chronic inflammation in both the hippocampus and the cortex. Furthermore, it increased AMPK activation and inhibited P65, NF-kB, mTOR, S6K activation and reduced BACE1 protein expression (54).
Administration of metformin for one year (orally:1 and 10mg/kg daily) to ovariectomized aged mice resulted in improved working memory and increased life span (55). On a molecular level, metformin increased the level of Brain-Derived Neurotrophic Factor (BDNF) (55). Similarly, 6 months treatment with metformin prevented spatial memory impairment in male mice consuming a high-fat diet (56). On a molecular level however, metformin resulted in a decreased transcription of neurotrophic factors and antioxidant regulator Nrf2 with no alterations in proteins levels (56). These two aforementioned studies reported improved cognition in the metformin group (55, 56), however the conflicting findings on molecular changes are not well understood. It is possible that metformin has positive effects on cognition but may result in mixed beneficial and detrimental biochemical alterations. The factors that lead to opposite molecular findings are not fully understood and need further investigations. Wang et al (57) reported that metformin enhanced spatial memory formation in adult mice via an activation of an atypical Protein-Kinase C-CREB-Binding Prtoein (aPKC-CBP) pathway that promotes hippocampal neurogenesis (57). The authors also reported a similar action of metformin on neurogenesis in rodents and human cell cultures (57). In a study comparing metformin to donepezil, metformin induced more hippocampal neurogenesis, resulting in improved spatial memory in AlCl3-induced mouse model of neurodegeneration (58). However, hippocampal neurogenesis was not involved in the improved cognitive function related to metformin administration to late-middle aged male mice (C57BL6/J) in a recent study by Kodali and colleagues (59) where microglia, proinflammatory cytokines and autophagy regulation were the identified mechanisms for the cognitive benefits related to metformin use (59). In a male rat model of prediabetes without obesity, non-hypoglycemic doses of metformin restored cerebrovascular and hippocampal alterations and improved cognitive function (60). In rats consuming a high-fat diet (61), metformin administration (21 days of 15 mg/kg BW twice daily) improved learning abilities. On a molecular level, metformin improved peripheral insulin sensitivity and brain mitochondrial dysfunction (61). Other studies reported that metformin combined with another drug has better effects on cognition. For example, a codelivery of metformin with phosphatidylserine liposome neuroprotectant improved learning and memory, decreased cytokine levels of Interleukin 1-β (IL1-β), Tumor Necrosis Factor- α (TNF-α), and Transforming Growth Factor β (TGF-β) in hippocampal tissues of rats with AD (62). In another study, metformin combination with fluoxetine in rats exposed to chronic stress and a high-fat diet resulted in improved memory and a decrease in hippocampal c-Jun expression (63). Finally, metformin combination with GLP-1ra enhanced learning and memory in high-fat fed mice whereas metformin alone had no effects on cognitive function (64).
In a study in intracerebroventricularly (ICV) streptozotocin (STZ)-injected mice impaired learning and memory functions were improved by metformin treatment (65). ICV-STZ injection or intranasal/oral metformin treatments had no effect on blood glucose concentrations. Intranasal treatment yielded higher concentration of metformin in the hippocampus and lower in the plasma compared to oral treatment. ICV-STZ injection and metformin treatments did not change amyloid beta-42 concentration in the hippocampus of mice. In hippocampal and cortical tissues of ICV-STZ-induced AD mice, insulin receptor (IR) and Akt expressions were unchanged, while phosphorylated insulin receptor (pIR) and pAkt expressions decreased compared to control. Metformin treatments did not change IR and Akt expressions but increased pIR and pAkt expressions. A study in a rat model of AD explored the therapeutic mechanism of metformin and its solid-lipid nanoformulation (SLN) using a microemulsion method. AD was induced with ICV-Abeta whereas the control-group (sham) received ICV-NS (66). Treatment arms included, disease-control (no treatment), metformin (50 mg/kg, 100 mg/kg and 150 mg/kg), SLN-metformin 50 mg/kg and memantine 1.8 mg/kg (positive-control). Abeta (1-42), hyperphosphorylated tau, markers of insulin signaling (pAKTser473, GSK-3beta, p-ERK), metformin level, neuronal injury score, Bcl2 and Bax, were evaluated in isolated brain after 21 days of treatment. Compared to sham, the disease-control group showed significantly higher memory impairment, hyperphosphorylated tau, Abeta (1-42), neuronal-injury, Bax and lower Bcl-2 expression. Treatment with metformin and nanoformulation significantly reversed these parameters. The AKT-ERK-GSK3beta-Hyperphosphorylated tau pathway was found to be involved in the protective effect of metformin.
Other studies reported that metformin improved cognition in other settings such as in diabetic epileptic rats (67), hypobaric hypoxic rats (68), methamphetamine induced neurodegeneration in male rats (69), mice treated with chemotherapy agents such as cyclophosphamide (70) or cisplatin (71), rats or mice with scopolamine induced learning and memory impairment (72, 73), neonatal hypoxia-ischemia rat model (74), rats with induced cerebral ischemia (75).
Human studies
Observational studies showing adverse or null associations
Clinical Outcomes
A large population-based case-control study of adults ≥ 65 years old compared the use of metformin between 7,086 cases with AD and 7,086 controls without AD using administrative data from the United Kingdom General Practice Research Database (GPRD) to define all variables. History of 60 or more prescriptions of metformin was associated with slightly higher risk of developing AD after adjustment for use of other diabetes medications, smoking, body mass index, hypertension, dyslipidemia, and use of angiotensin converting enzyme inhibitors and statins. (OR = 1.71, 95% CI = 1.12–2.60) (76). The authors concluded that the study did not provide evidence that the use of metformin reduces the risk of AD and suggested that the use of metformin could be related to a higher risk of AD. It is important to point out that this study included persons without diabetes, who comprised most of the sample. In addition, the higher odds of metformin use among persons with AD compared to those without AD was apparent in the adjusted model but not in the unadjusted model (OR = 1.06, 95% CI = 0.77-1.46). In another analysis examining an exposure variable defined by a history of diabetes treated with metformin only (no other diabetes medications), the odds of metformin use among cases with AD was not significantly higher than in controls without AD. An Australian cross-sectional study also examined the association of metformin with cognitive impairment combining samples from 4 sources that included persons with AD and MCI: the Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging, the Research in Memory (PRIME) clinics study, the Cognitive, Dementia, and Memory Services from the Barwon region of southeastern Australia, and persons with AD from private geriatric practices. The final analytic sample included 1354 persons, 126 with diabetes, with a mean age of 73.8 years (77). Levels of cognitive performance were defined by performance in the mini-mental status exam (MMSE). MMSE < 18 was classified as most impaired, 18 to 23 as mildly impaired, 24 to 27 as minimally impaired, and 28 or more as not impaired. In analyses using ordinal logistic regression a history of diabetes was related to a higher risk of cognitive impairment (OR = 1.51; 95% CI = 1.03-2.21) after adjustment for age, sex, education, and history of depression. In an analysis restricted to the 126 participants with diabetes, metformin use was related to a higher risk of cognitive impairment after adjustment for age, sex, education, and history of depression. (OR=2.23, 95% CI = 1.05-4.75). This association was partially attenuated by adjustment for cobalamin levels. The investigators concluded that persons with diabetes taking metformin should be monitored for cognitive impairment. Kuan et al (78) found that compared to persons not taking metformin, those taking metformin had an increased risk of all cause dementia (HR=1.66, 95% CI= 1.35-2.04), AD (HR=2.13, 05%CI= 1.20-3.79) and vascular dementia (HR=2.30, 95%CI=1.25-4.22) over 12 years follow-up in a cohort study of persons with T2D from the Taiwan’s National Health Insurance Research Database (78). The definition of dementia and dementia subtypes was based on administrative data. A community-based cross-sectional study in 4,160 adults with and without hyperglycemia without dementia and a mean age of 74.1 years from the Trinity, Ulster and Department of Agriculture Study examined the association of metformin use and cognitive performance, assessed with the repeatable battery for assessment of neuropsychological statust (RBANS) (79). Compared with persons without hyperglycemia, persons with hyperglycemia using metformin had a higher risk of lower performance in the RBANS (OR = 1.36; 95% CI – 1.03, 1.80) as well as with higher risk of vitamin B12 and B6 deficiency. A prospective cohort of older adults (n=732, mean age=76.6 years) by Koo and colleagues reported that among persons with T2D metformin use was associated with worse scores on MMSE and verbal immediate recall, and no association with the Korean Consortium to Establish a Registry for Alzheimer’s Diseases Assessment (CERAD) or activities of daily living after 2.9 years follow-up (80). A single hospital retrospective cohort study in Northern Taiwan used administrative and hospital record data (81) to compare dementia risk among 67,281 persons with T2D aged approximately 62 years taking and not taking DPP-4i and found a higher risk of dementia (hazard ratio = 1.11; 95% confidence interval, 1.06-1.15, adjusting for age, sex, and comorbidiites) among persons taking both metformin and a DPP-4i. The Diabetes Prevention Program (DPP) was a randomized trial comparing metformin, lifestyle intervention, and placebo in persons at risk for T2D that has continued in an observational phase called the DPP Outcomes Study (DPPOS). The DPP reported that the risk of T2D was significantly decreased in the lifestyle and metformin arms compared with placebo after 3 years (82). Cognitive performance was tested in DPPOS approximately 10 years after randomization in 2,280 DPP aged 63 years on average at the time of cognitive assessment. Tests included the Spanish English Verbal Learning Test, the Digit Symbol Substitution Test, and letter and category fluency tests (83). Cognitive performance was similar across the metformin, lifestyle, and placebo arms. In addition, cumulative metformin use, including study metformin and metformin prescribed for participants who developed T2D in the placebo and lifestyle arms, was not associated with cognitive performance. Compared to other observational studies, the DPP study had the most rigorous assessment of exposure and outcomes, although the cognitive assessment was conducted approximately a decade after randomization.
Biomarker and Neuropathology outcomes
There is a dearth of studies that have examined metformin use and neuropathology outcomes measured with brain imaging, blood biomarkers or post-mortem neuropathology. A recent large prospective community-based observational study of older subjects without dementia, from the Sydney Memory and Ageing Study (n=1037, age =70-90 years) reported no association between metformin use and the rate of changes in brain volume over 2 years (84). A post-mortem case-control study of persons with and without diabetes (n=248, mean age at death=81.2 years) from the Mount Sinai School of Medicine Brain Bank reported fewer neuritic plaques in persons who were taking a combination of insulin and any oral antidiabetic medication compared to those taking only one drug (either insulin or an oral drug) (85). No differences were found for neurofibrillary tangles (85).
Observational studies showing associations with improved outcomes
Clinical outcomes
The following are examples of studies showing an association of metformin use with lower risk of cognitive impairment and dementia. In a retrospective cohort study of persons with T2D aged approximately 63 years comparing 15,676 metformin users to 15,676 non-users Chin-Hsiao et al reported that metformin used was associated with a lower risk of dementia after two years of follow-up (HR=0.70, 95%CI=0.63-0.79) (86). In another retrospective study of 127,209 persons with T2D aged 50 years and older using administrative data from Taiwan’s National Health Insurance database (87), Hsu et al found that metformin was associated with lower risk of dementia (OR=0.76, 95% CI=0.58-0.98). Furthermore, the combination of metformin with sulfonylureas was associated with a lower risk of dementia over 8 years. Long-term use of metformin was related to lower risk of cognitive impairment (defined by a MMSE < 24) over 4 years of follow-up in a population-based study of 365 adults aged approximately 66 years with T2D from the Singapore Longitudinal Aging Study (OR = 0.49, 95% CI 0.25–0.95) (88). Similar findings were reported by Samaras and colleagues in 1,037 participants without dementia in the Sydney Memory and Aging Study (84). Persons with T2D who took metformin had slower global cognition and executive function decline over 6 years compared to persons with T2D who did not take metformin. Those not taking metformin showed higher risk of incident dementia (OR=5.29, 95% CI=1.17-23.88) compared to those taking metformin (84). The Personality and Total Health (PATH) Through Life Study reported that among 113 participants with diabetes taking oral diabetes medications, metformin use was associated with better cognitive performance in the domains of verbal learning, working memory and executive function (89). A retrospective cohort study using administrative data from 28,640 persons with T2D 65 years and older from the Veterans Administration system in the United States used inverse probability of treatment weighting to adjust for confounding by indication comparing the risk of dementia between users of metformin and users of sulfonylureas (90). Metformin use of at least 2 years duration was associated with a lower risk of dementia compared to sulfonylurea during 5 years of follow-up in persons < 75 years old (HR = 0.89, 95% CI = 0.79-0.89). A case-control study in Germany using an administrative database compared the use of metformin between 8,276 persons with T2D and dementia and 8,726 persons with T2D without dementia matched by demographics and other relevant variables. Metformin use as monotherapy (OR = 0.71, 95% CI = 0.66-0.76) or in combination with sulfonylurea (OR = 0.90; 95% CI = 0.89-0.92) was associated with a decreased risk of dementia (91). A study in the Alzheimer’s disease neuroimaging initiative (ADNI) compared cognitive performance among 810 persons with mild cognitive impairment (MCI) grouped by the presence of T2D and/or metformin treatment (92). The groups were paired by age, gender, education, and APOE status. Composite cognitive test scores and cognitive change in those with T2D treated with metformin were comparable to those without T2D and better than those with T2D not treated with metformin. There were similar findings for hippocampal volume and cortical thickness in AD signature areas. Another study in an administrative dataset from the United States (OPTUM HER) compared the risk of dementia between 96,140 new users of metformin and 16,451 new users of sulfonylureas with a mean age of 66 years (93). During 5 years of follow up, using IPTW to adjust for confounding by indication, the risk of dementia was lower in persons using metformin compared to those using sulfonylurea (HR = 0.80, 95% CI 0.73 to 0.88). A cohort study in 210,237 persons with T2D in the United Kingdom Clinical Practice Research Datalink (94) showed that compared with metformin initiators (n = 114,628), patients who received no diabetes medication (n = 95,609) had lower risk of dementia compared to non-users (adjusted hazard ratio = 0.88 [95% confidence interval: 0.84-0.92] and 0.90 [0.84-0.96]) in analyses adjusted for a propensity score, despite persons not on pharmacological treatment having better glycemia measured with Hemoglobin A1C. A study using the Taiwan National Insurance Research Database (95) examined the risk of dementia in 31,384 pairs with T2D that differed by metformin adherence and were matched by propensity score during 5 years of follow-up. Metformin adherence was associated with a lower risk of dementia (HR = 0.72, p <0.001).
Biomarker outcomes
In a cross-sectional study of late middle-aged adults without dementia (n=350, mean age= 64.15 years) where metformin was the most commonly used medication (85.18 % of all diabetes medications) diabetes medication use was associated with lower brain Aβ burden as measured on Positron Emission Tomography imaging (96). In an analysis of older adults without dementia (n=900, mean age = 73.54) from the Alzheimer’s Disease Neuroimaging Initiative (ADNI), McIntosh and colleagues, reported that untreated persons with T2D had higher CSF levels of p-tau, t-tau, and p-tau/Aβ1-42 when compared to treated persons with T2D, persons with euglycemia or with pre-diabetes (24). Although the study did not examine individual medications due to sample size and heterogeneity, metformin was the most commonly used diabetes medication in the study (24).
Meta-analyses of observational studies relating metformin and cognitive outcomes
Several meta-analyses have reported that metformin use is not associated with better cognitive outcomes. In a meta-analysis of observational studies examining the association between metformin and neurodegenerative diseases (19 studies, n=285 966), Ping and colleagues (97) reported a null association between metformin exposure and the incidence of overall neurodegenerative diseases (OR=1.04, 95% CI= 0.92-1.17) including AD (OR=0.96, 95% CI=0.85-1.08). Metformin exposure however was related to higher risk of developing Parkinson disease (OR=1.66, 95% CI=1.14-2.42). Similarly, a meta-analysis of 6 studies (n=544, 093) found no association of metformin with the incidence of dementia, although an association with lower dementia risk was nearly statistically significant (RR=0.79, 95% CI=0.62-1.01, p=0.064) (98). A pooled analysis from 5 major population based-cohorts also reported a null association of metformin with new-onset dementia and global cognitive function (99). A meta-analysis by Luo et (100) included 10 observational studies with 229,110 patients and found no significant association between metformin exposure and AD incidence (OR 1.17, 95% CI 0.88-1.56) but found an association between metformin exposure and higher AD risk among Asians (OR 1.71, 95% CI 1.24-2.37).
Other meta-analyses have reported an association between metformin and better cognitive outcomes. Campbell et al reported that metformin use was associated with less cognitive impairment in persons with T2D (OR=0.55, 95%CI= 0.38-0.78) (101, 102) in three combined cross-sectional studies. Similarly, metformin use was associated with a lower incidence of dementia (HR = 0.76, 95% CI 0.39 to 0.88) in six combined longitudinal studies, according to the same meta-analysis (101, 102). A metanalysis by Ji et all using 14 observational studies including 396,332 participants (103) found that metformin exposure was associated with a lower risk of all cause dementia (RR =0.79, 95% CI: 0.68,0.91) but found no association in participants of European descent (RR = 1.01, 95% CI 0.66-1.54). Lastly, Zhang et al conducted a metanalysis relating metformin exposure to neurodegenerative diseases including AD/ADRD in 12 cohort studies including 194,792 participants with T2D. There was an inverse association between metformin exposure and neurodegenerative diseases (pooled relative risk = 0.77, 95 CI 0.67-0.88) (104). This association was more prominent for metformin exposure of 4 years or longer. It is important to point out that the majority of studies reviewed in meta-analyses used administrative data to ascertain metformin exposure and the cognitive outcomes.
Clinical trials
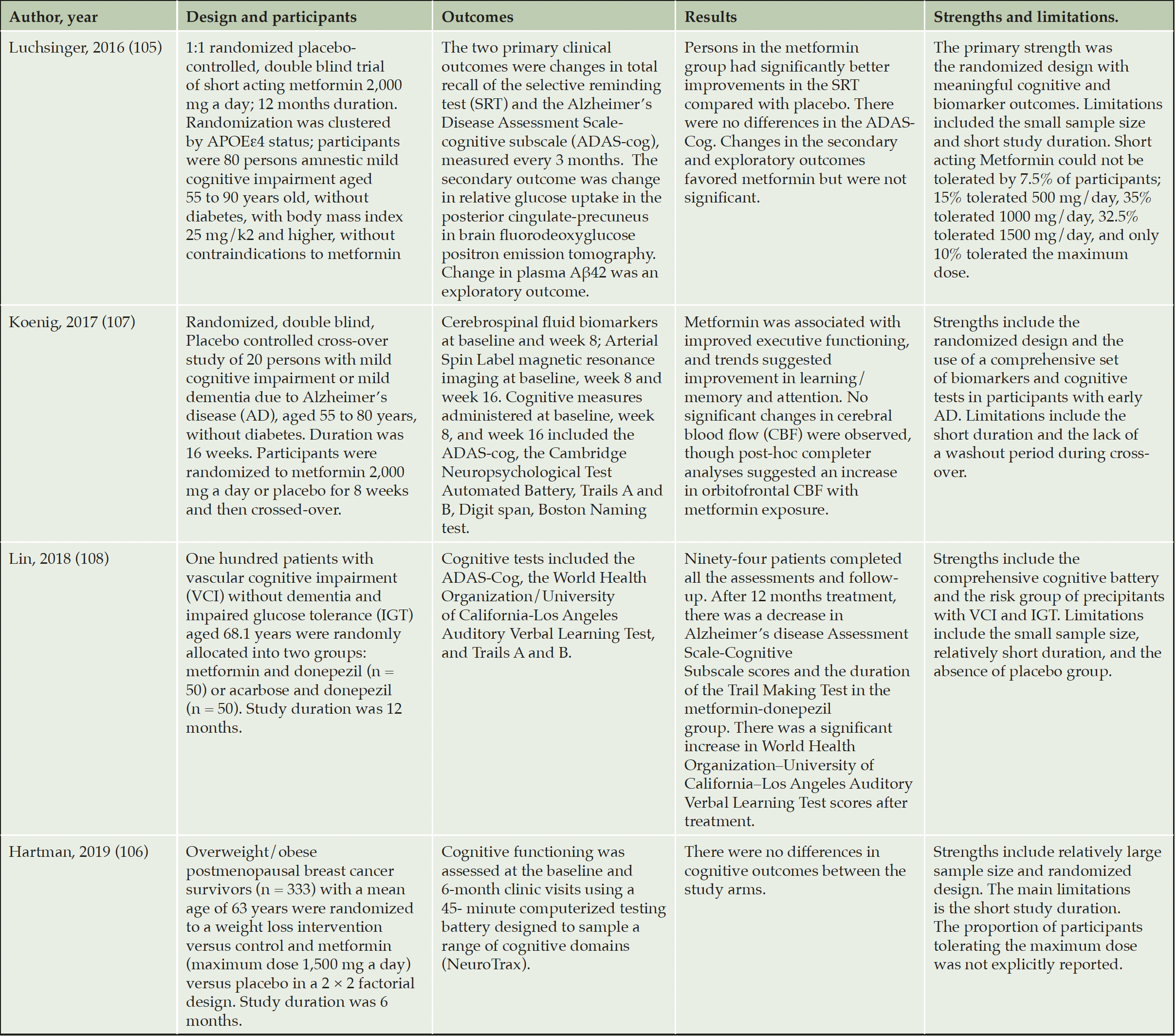
Few clinical trials of metformin with primary cognitive outcomes have been conducted so far (Table 1). In a small one-year pilot randomized clinical trial (RCT) in subjects with amnestic mild cognitive impairment without T2D (n= 80, mean age= 65 years), randomization to 1000 mg of metformin twice daily was safe and feasible and resulted in better performance in memory, measured by the total recall in the Buschke selective reminding test compared to the placebo group (105). Biomarker outcomes including plasma amyloid β42/40 ratio and FDG PET uptake in regions related to AD favored the metformin arm but were not significant compared with placebo. A six month randomized controlled trial examining the effects of metformin (1500 mg/day) and weight loss intervention on cognitive function among breast cancer survivors (n=333, mean age =62.6 years) found no significant difference on cognitive domains between metformin and placebo (106). In An eight-week crossover RCT in persons with cognitive impairment due to AD without T2D (n=20) metformin improved executive function, but its beneficial effect on learning/memory and attention was close to statistical significance (p=0.06, p=0.07 respectively) (107). The same study reported no differences in CSF measures of Aβ-42, total tau, P-tau while an increase in cerebral blood flow using Arterial Spin Label MRI in orbitofrontal regions was observed in the metformin group (107). Lastly, metformin therapy in conjunction with donepezil improved cognitive function compared to the acarbose-donepezil group after one-year RCT among subjects with non-dementia vascular cognitive impairment (n=100, mean age ≈ 66 years) (108).
One study simulated a clinical trial of metformin vs. sulfonylurea in persons with T2D, aged 50 years and older, without prior diabetes treatment, without MCI or dementia at baseline, using electronic medical records from the United States Research Patient Data Registry (13,191 patients) and the United Kingdom Clinical Practice Research Datalink (108,025 patients) (109). It found that the risk of dementia in persons on metformin was lower than those on sulfonylurea, but this effect dissipated over time.
Caveats and limitations of reported studies
Overall, non-human and human studies relating metformin to cognitive and brain outcomes have inconsistent results. For non-human studies, studies have been reported across many different models showing inconsistent mechanisms for both potential harm and benefit. This could reflect the pleotropic effects of metformin but could also demonstrate the uncertainty about the relevance of the animal models used to the effects of metformin in humans.
Observational studies are subject to various biases and confounding. Most observational studies examining the association of metformin with dementia do not clearly assess time-related biases (110). In addition, they are subject to confounding by indication, that is, that persons with cognitive impairment are more likely to use metformin because of their cognitive status and related comorbididites rather than metformin having an effect on cognition. The few observational studies that have used propensity scores and techniques to account for confounding by indication such as IPTW reported an association between metformin exposure and lower risk of cognitive impairment including dementia. Observational studies are mostly limited to persons with T2D, who are subject to confounding by medications and comorbidities. This confounding can be partially adjusted by propensity scores and IPTW, but these techniques cannot completely eliminate unmeasured or residual confounding. Lastly, the majority of observational studies have ascertained metformin exposure and the cognitive outcomes (e.g. dementia) using administrative data, which is subject to measurement bias. The only way to overcome the limitations of observational studies is by conducting clinical trials.
The existing clinical trials are small and of relatively short duration and are thus subject to chance findings. In addition, they are limited to persons without T2D to avoid confounding by the effects of diabetes medications other than metformin. There is a need to conduct clinical trials of metformin vs. placebo of several years duration that include cognitive and biomarker outcomes. Other unanswered questions that need to be addressed in future studies include metformin dosage and side effects and the role of sex and genetics in the associations between metformin and cognitive function (111). A detailed discussion of some of the important gaps on metformin and aging was thoroughly discussed in a recent review by Wang, et al (111). One of the issues highlighted was finding the best dose of metformin that is both neuroprotective and has minimal side effects. This is crucial to ensure a benefit and long-term compliance. It should be noted that metformin has been used in clinical practice as first line therapy for T2D for decades and has been shown to be safe. Some side effects of metformin such as vitamin B12 deficiency that could affect cognition might be prevented by vitamin supplementation (112).
Ongoing studies
There are several ongoing observational studies and clinical trials that seek to clarify the association of metformin with AD/ADRD and the underlying mechanisms of this association if it exists.
The Diabetes Prevention Program Outcomes Study (DPPOS) AD/ADRD project (National Institutes of Health [NIH] grant U19AG078558) is conducting comprehensive phenotyping of cognitive syndromes (mild cognitive impairment, dementia) using the National Alzheimer’s Coordinating Center Uniform Dataset version 3 and AD pathology using plasma biomarkers of amyloid, tau, and neurodegeneration in a cohort of over 1800 persons with pre-diabetes and T2D. In addition, a sub-sample of this cohort is undergoing amyloid PET and brain MRI. Although the DPPOS AD/ADRD is an observational study it will overcome some of the caveats mentioned before because it will have detailed ascertainment of metformin exposures, comorbidities, other medications, and state of the art AD/ADRD phenotyping. The DPPOS has over 20 years of detailed data on metformin exposure. Randomized trials of metformin vs. placebo in persons with T2D are not feasible since use of metformin in T2D as first line therapy is standard of care.
There are several ongoing or planned clinical trials. Metformin in Alzheimer’s dementia Prevention (MAP, NIH grant R01AG062624, clinicaltrials.gov ID NCT04098666) is an ongoing multisite 18 month 1:1 randomized placebo controlled clinical trial of extended-release metformin 2000 mg in 326 persons with amnestic MCI without T2D in the United States. It includes cognitive outcomes and biomarker outcomes, including measures of neurodegeneration and cerebrovascular disease on brain MRI, amyloid and tau in brain PET, and plasma biomarkers of amyloid, tau, and neurodegeneration. MAP is planned for completion in 2026. Preventing Cognitive Decline with Metformin (MetMemory Study, clinicaltrials.gov ID NCT04511416) is a 3-year randomized clinical trial of extended release metformin vs. placebo in 242 persons with mild cognitive impairment with a hemoglobin A1C < 6.5% with cognitive and biomarker outcomes based currently under way in Australia. METformin and Finger Intervention to Prevent Cognitive Impairment and Disability in Older Adults at Risk for Dementia (MET-FINGER, NCT05109169) is a 24 month 1:1:1 randomized trial of metformin 2000 mg/day vs. 1000 mg/day vs placebo to be conducted in the United Kingdom, Finland, and Sweden. Six hundred persons at risk for dementia will be randomized 1:1 to the FINGER 2.0 intervention vs. comparator intervention; those in the FINGER 2.0 group at increased risk for diabetes will be randomized to the metformin arms.
These studies will provide important data on the cognitive safety of metformin and its effects on cognitive outcomes, brain structure, and AD biomarkers both in persons with and without T2D.
Conclusion
Metformin is a safe and effective treatment for T2D, also used for the prevention of T2D, that has multiple metabolic benefits that have been hypothesized to ameliorate diseases such as cancer and diseases related to aging in general. Studies in animal models and observational studies in humans suggest that metformin is beneficial for dementia prevention, but this is countered by non-human and human studies that suggest that metformin could be harmful from a cognitive and neuropathology standpoint. A few small randomized clinical trials in persons without T2D suggest that metformin could be beneficial in preventing cognitive impairment in persons with prodromal dementia or early dementia. It is necessary to conduct observational and mechanistic studies in persons with T2D with appropriate ascertainment of metformin and covariates, and state of the art phenotyping of AD/ADRD. Moreover, it is necessary to conduct placebo-controlled randomized clinical trials of metformin in large samples of persons without T2D with relatively long duration and state of the art AD/ADRD phenotyping. Metformin could also be tested in combination with other strategies.
Acknowledgements: The authors wish to acknowledge the support of the National Institute on Aging from the National Institute of Health in the United States and the Alzheimer’s Disease Drug Discovery Foundation for some of the studies cited in this review. Dr. Luchsinger receives a stipend from Wolters Kluwer as an Editor in Chief of the journal Alzheimer’s Disease and Associated Disorders. He has been a consultant to Merck aKA in work related to metformin and currently receives extended release metformin and matching placebo from EMD Serono, subsidiary of Merck, for a clinical trial of metformin in amnestic mild cognitive impairment.
Funding: Dr. Luchsinger’s work in this review was supported by NIH grants R01AG062624, U19AG078558, and K24AG045334.
Conflict of interest: Dr. Luchsinger has been a consultant to Merck KGaA; the project funded by grant R01AG062624 receives metformin and matching placebo from EMD Serono, a business of Merck KGaA.
References
1. Biessels, G.J. and F. Despa, Cognitive decline and dementia in diabetes mellitus: mechanisms and clinical implications. Nat Rev Endocrinol 2018; 14: p. 591-604.
2. Chatterjee, S. and A. Mudher, Alzheimer’s Disease and Type 2 Diabetes: A Critical Assessment of the Shared Pathological Traits. Front Neurosci 2018; 12: p. 383.
3. Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016.
4. Raz, L., J. Knoefel, and K. Bhaskar, The neuropathology and cerebrovascular mechanisms of dementia. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 2016; 36: p. 172-186.
5. Pasqualetti, G., P. Brooks Dj Fau – Edison, and P. Edison, The role of neuroinflammation in dementias. 2015.
6. Luca, M., A. Luca, and C. Calandra, The Role of Oxidative Damage in the Pathogenesis and Progression of Alzheimer’s Disease and Vascular Dementia. Oxidative Medicine and Cellular Longevity 2015; 2015: p. 504678.
7. Saez-Atienzar, S. and E. Masliah, Cellular senescence and Alzheimer disease: the egg and the chicken scenario. Nature Reviews Neuroscience 2020; 21: p. 433-444.
8. Sun, Y., et al., Metabolism: A Novel Shared Link between Diabetes Mellitus and Alzheimer’s Disease. Journal of Diabetes Research 2020; 2020: p. 4981814.
9. Akter, K., et al., Diabetes mellitus and Alzheimer’s disease: shared pathology and treatment? British journal of clinical pharmacology 2011; 71: p. 365-376.
10. Boccardi, V., I. Murasecco, and P. Mecocci, Diabetes drugs in the fight against Alzheimer’s disease. Ageing Res Rev 2019; 54: p. 100936.
11. Jack, C.R., Jr., et al., NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement 2018; 14: p. 535-562.
12. Abner, E.L., et al., Diabetes is associated with cerebrovascular but not Alzheimer’s disease neuropathology. Alzheimer’s & dementia : the journal of the Alzheimer’s Association 2016; 12: p. 882-889.
13. Dos Santos Matioli, M.N.P., et al., Diabetes is Not Associated with Alzheimer’s Disease Neuropathology. Journal of Alzheimer’s disease : JAD 2017; 60: p. 1035-1043.
14. Pruzin, J.J., et al., Diabetes, Hemoglobin A1C, and Regional Alzheimer Disease and Infarct Pathology. Alzheimer Dis Assoc Disord 2017; 31: p. 41-47.
15. Crane, P.K., et al., Glucose levels during life and neuropathologic findings at autopsy among people never treated for diabetes. Neurobiology of aging 2016; 48: p. 72-82.
16. Alafuzoff, I., et al., Beta-amyloid deposition in brains of subjects with diabetes. Neuropathol Appl Neurobiol 2009; 35: p. 60-68.
17. Arvanitakis, Z., et al., Diabetes is related to cerebral infarction but not to AD pathology in older persons. Neurology 2006; 67: p. 1960-1965.
18. Thambisetty, M., et al., Glucose intolerance, insulin resistance, and pathological features of Alzheimer disease in the Baltimore Longitudinal Study of Aging. JAMA Neurol 2013; 70: p. 1167-1172.
19. Gottesman, R.F., et al., Association Between Midlife Vascular Risk Factors and Estimated Brain Amyloid Deposition. JAMA 2017; 317: p. 1443-1450.
20. Moran, C., et al., Type 2 diabetes mellitus and biomarkers of neurodegeneration. Neurology 2015; 85: p. 1123-1130.
21. Roberts, R.O., et al., Diabetes and elevated hemoglobin A1c levels are associated with brain hypometabolism but not amyloid accumulation. J Nucl Med 2014; 55: p. 759-764.
22. Groeneveld, O.N., et al., The Clinical Phenotype of Vascular Cognitive Impairment in Patients with Type 2 Diabetes Mellitus. Journal of Alzheimer’s Disease 2019; 68: p. 311-322.
23. Peila, R., et al., Type 2 diabetes, APOE gene, and the risk for dementia and related pathologies: The Honolulu-Asia Aging Study. Diabetes 2002; 51: p. 1256-1262.
24. McIntosh, E.C. and D.A. Nation, Importance of Treatment Status in Links Between Type 2 Diabetes and Alzheimer’s Disease. Diabetes Care 2019; 42: p. 972.
25. Kim, I., et al., A relationship between Alzheimer’s disease and type 2 diabetes mellitus through the measurement of serum amyloid-beta autoantibodies. J Alzheimers Dis 2010; 19: p. 1371-1376.
26. Ahtiluoto, S., et al., Diabetes, Alzheimer disease, and vascular dementia: a population-based neuropathologic study. Neurology 2010; 75: p. 1195-1202.
27. Beeri, M.S., et al., Type 2 diabetes is negatively associated with Alzheimer’s disease neuropathology. J Gerontol A Biol Sci Med Sci 2005; 60: p. 471-475.
28. Li, W., et al., Type 2 diabetes mellitus and cerebrospinal fluid Alzheimer’s disease biomarker amyloid beta1-42 in Alzheimer’s Disease Neuroimaging Initiative participants. Alzheimers Dement (Amst) 2018; 10: p. 94-98.
29. Peters, K.E., et al., Plasma Amyloid-beta Peptides in Type 2 Diabetes: A Matched Case-Control Study. J Alzheimers Dis 2017; 56: p. 1127-1133.
30. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes-2019.
31. Taylor, R., Type 2 Diabetes. Diabetes Care 2013; 36: p. 1047.
32. Verma, S. and J.J.V. McMurray, SGLT2 inhibitors and mechanisms of cardiovascular benefit: a state-of-the-art review.
33. American Diabetes, A., 9. Pharmacologic Approaches to Glycemic Treatment: <em>Standards of Medical Care in Diabetes—2021</em>. Diabetes Care 2021; 44: p. S111.
34. Flory, J. and K. Lipska, Metformin in 2019.
35. Whitmer, R.A., et al., Hypoglycemic episodes and risk of dementia in older patients with type 2 diabetes mellitus.
36. Lebovitz, H.E., Thiazolidinediones: the Forgotten Diabetes Medications.
37. Liu, H., et al., The protective role of DPP4 inhibitors in atherosclerosis.
38. Aroda, V.A.-O., A review of GLP-1 receptor agonists: Evolution and advancement, through the lens of randomised controlled trials.
39. Bendlin, B.B., Antidiabetic therapies and Alzheimer disease. Dialogues in clinical neuroscience 2019; 21: p. 83-91.
40. Lv, Z. and Y. Guo, Metformin and Its Benefits for Various Diseases. Front Endocrinol (Lausanne) 2020; 11: p. 191.
41. Chen, Y., et al., Antidiabetic drug metformin (GlucophageR) increases biogenesis of Alzheimer’s amyloid peptides via up-regulating BACE1 transcription. Proceedings of the National Academy of Sciences of the United States of America 2009; 106: p. 3907-3912.
42. DiTacchio, K.A., S.F. Heinemann, and G. Dziewczapolski, Metformin treatment alters memory function in a mouse model of Alzheimer’s disease. J Alzheimers Dis 2015; 44: p. 43-48.
43. Li, J., et al., Metformin attenuates Alzheimer’s disease-like neuropathology in obese, leptin-resistant mice. Pharmacol Biochem Behav 2012; 101: p. 564-574.
44. McNeilly, A.D., et al., A high-fat-diet-induced cognitive deficit in rats that is not prevented by improving insulin sensitivity with metformin. Diabetologia 2012; 55: p. 3061-3070.
45. Thangthaeng, N., et al., Metformin Impairs Spatial Memory and Visual Acuity in Old Male Mice. Aging Dis 2017; 8: p. 17-30.
46. Picone, P., et al., Metformin increases APP expression and processing via oxidative stress, mitochondrial dysfunction and NF-kappaB activation: Use of insulin to attenuate metformin’s effect. Biochim Biophys Acta 2015; 1853: p. 1046-1059.
47. Picone, P., et al., Biological and biophysics aspects of metformin-induced effects: cortex mitochondrial dysfunction and promotion of toxic amyloid pre-fibrillar aggregates. Aging (Albany NY) 2016; 8: p. 1718-1734.
48. Barini, E., et al., Metformin promotes tau aggregation and exacerbates abnormal behavior in a mouse model of tauopathy. Mol Neurodegener 2016; 11: p. 16.
49. Alzoubi, K.H., et al., Metformin Eased Cognitive Impairment Induced by Chronic L-methionine Administration: Potential Role of Oxidative Stress. Curr Neuropharmacol 2014; 12: p. 186-192.
50. Zhao, R.R., et al., Metformin protects against seizures, learning and memory impairments and oxidative damage induced by pentylenetetrazole-induced kindling in mice. Biochem Biophys Res Commun 2014; 448: p. 414-417.
51. Farr, S.A., et al., Metformin Improves Learning and Memory in the SAMP8 Mouse Model of Alzheimer’s Disease. J Alzheimers Dis 2019; 68: p. 1699-1710.
52. Chen, F., et al., Antidiabetic drugs restore abnormal transport of amyloid-β across the blood-brain barrier and memory impairment in db/db mice. Neuropharmacology 2016; 101: p. 123-136.
53. Zhang, J., et al., Metformin treatment improves the spatial memory of aged mice in an APOE genotype-dependent manner. FASEB J 2019; 33: p. 7748-7757.
54. Ou, Z., et al., Metformin treatment prevents amyloid plaque deposition and memory impairment in APP/PS1 mice. Brain Behav Immun 2018; 69: p. 351-363.
55. Zakeri, M., et al., Pro-neurocognitive and anti-sarcopenic benefits of one-year metformin therapy in ovariectomized aged mice. Clin Exp Pharmacol Physiol 2019; 46: p. 1133-1140.
56. Allard, J.S., et al., Prolonged metformin treatment leads to reduced transcription of Nrf2 and neurotrophic factors without cognitive impairment in older C57BL/6J mice. Behav Brain Res 2016; 301: p. 1-9.
57. Wang, J., et al., Metformin activates an atypical PKC-CBP pathway to promote neurogenesis and enhance spatial memory formation. 2012.
58. Ahmed, S., et al., Effect of Metformin on Adult Hippocampal Neurogenesis: Comparison with Donepezil and Links to Cognition. J Mol Neurosci 2017; 62: p. 88-98.
59. Kodali, M., et al., Metformin treatment in late middle age improves cognitive function with alleviation of microglial activation and enhancement of autophagy in the hippocampus. Aging Cell 2021: p. e13277.
60. Fakih, W., et al., Dysfunctional cerebrovascular tone contributes to cognitive impairment in a non-obese rat model of prediabetic challenge: Role of suppression of autophagy and modulation by anti-diabetic drugs. Biochem Pharmacol 2020; 178: p. 114041.
61. Pintana, H., et al., Effects of metformin on learning and memory behaviors and brain mitochondrial functions in high fat diet induced insulin resistant rats. Life Sci 2012; 91: p. 409-414.
62. Saffari, P.M., et al., Metformin loaded phosphatidylserine nanoliposomes improve memory deficit and reduce neuroinflammation in streptozotocin-induced Alzheimer’s disease model. Life Sci 2020; 255: p. 117861.
63. Khedr, S.A., et al., Metformin potentiates cognitive and antidepressant effects of fluoxetine in rats exposed to chronic restraint stress and high fat diet: potential involvement of hippocampal c-Jun repression. Naunyn Schmiedebergs Arch Pharmacol 2018; 391: p. 407-422.
64. Lennox, R., et al., Comparison of the independent and combined effects of sub-chronic therapy with metformin and a stable GLP-1 receptor agonist on cognitive function, hippocampal synaptic plasticity and metabolic control in high-fat fed mice. Neuropharmacology 2014; 86: p. 22-30.
65. Kazkayasi, I., et al., Intranasal metformin treatment ameliorates cognitive functions via insulin signaling pathway in ICV-STZ-induced mice model of Alzheimer’s disease. Life Sci 2022; 299: p. 120538.
66. Kumar, H., et al., Novel therapeutic mechanism of action of metformin and its nanoformulation in Alzheimer’s disease and role of AKT/ERK/GSK pathway. Eur J Pharm Sci 2023; 181: p. 106348.
67. Mohamed, M.A.E., et al., Metformin and trimetazidine ameliorate diabetes-induced cognitive impediment in status epileptic rats. Epilepsy Behav 2020; 104: p. 106893.
68. Zhao, M., et al., Metformin administration prevents memory impairment induced by hypobaric hypoxia in rats. Behav Brain Res 2019; 363: p. 30-37.
69. Keshavarzi, S., et al., Protective role of metformin against methamphetamine induced anxiety, depression, cognition impairment and neurodegeneration in rat: The role of CREB/BDNF and Akt/GSK3 signaling pathways. Neurotoxicology 2019; 72: p. 74-84.
70. Alhowail, A.H., et al., Ameliorative effect of metformin on cyclophosphamide-induced memory impairment in mice. Eur Rev Med Pharmacol Sci 2019; 23: p. 9660-9666.
71. Zhou, W., A. Kavelaars, and C.J. Heijnen, Metformin Prevents Cisplatin-Induced Cognitive Impairment and Brain Damage in Mice. PLoS One 2016; 11: p. e0151890.
72. Aksoz, E., et al., The protective effect of metformin in scopolamine-induced learning and memory impairment in rats. Pharmacol Rep 2019; 71: p. 818-825.
73. Mostafa, D.K., C.A. Ismail, and D.A. Ghareeb, Differential metformin dose-dependent effects on cognition in rats: role of Akt. Psychopharmacology (Berl) 2016; 233: p. 2513-2524.
74. Qi, B., et al., Metformin Attenuates Cognitive Impairments in Hypoxia-Ischemia Neonatal Rats via Improving Remyelination. Cell Mol Neurobiol 2017; 37: p. 1269-1278.
75. Ashabi, G., et al., Subchronic metformin pretreatment enhances novel object recognition memory task in forebrain ischemia: behavioural, molecular, and electrophysiological studies. Can J Physiol Pharmacol 2017; 95: p. 388-395.
76. Imfeld, P., et al., Metformin, other antidiabetic drugs, and risk of Alzheimer’s disease: a population-based case-control study. J Am Geriatr Soc 2012; 60: p. 916-921.
77. Moore, E.M., et al., Increased risk of cognitive impairment in patients with diabetes is associated with metformin. Diabetes Care 2013; 36: p. 2981-2987.
78. Kuan, Y.C., et al., Effects of metformin exposure on neurodegenerative diseases in elderly patients with type 2 diabetes mellitus. Prog Neuropsychopharmacol Biol Psychiatry 2017; 79: p. 77-83.
79. Porter, K.M., et al., Hyperglycemia and Metformin Use Are Associated With B Vitamin Deficiency and Cognitive Dysfunction in Older Adults. J Clin Endocrinol Metab 2019; 104: p. 4837-4847.
80. Koo, B.K., et al., Taking metformin and cognitive function change in older patients with diabetes. Geriatr Gerontol Int 2019; 19: p. 755-761.
81. Tzeng, I.S. and T.H. Hsieh, Collocation of metformin and dipeptidyl peptidase-4 inhibitor is associated with increased risk of diabetes-related vascular dementia: A single hospital study in Northern Taiwan. Expert Opin Investig Drugs 2023; 32: p. 171-176.
82. Reduction in the Incidence of Type 2 Diabetes with Lifestyle Intervention or Metformin. New England Journal of Medicine 2002; 346: p. 393-403.
83. Luchsinger, J.A., et al., Metformin, Lifestyle Intervention, and Cognition in the Diabetes Prevention Program Outcomes Study. Diabetes Care 2017; 40: p. 958-965.
84. Samaras, K., et al., Metformin Use Is Associated With Slowed Cognitive Decline and Reduced Incident Dementia in Older Adults With Type 2 Diabetes: The Sydney Memory and Ageing Study. Diabetes Care 2020; 43: p. 2691-2701.
85. Beeri, M.S., et al., Insulin in combination with other diabetes medication is associated with less Alzheimer neuropathology. 2008.
86. Chin-Hsiao, T., Metformin and the Risk of Dementia in Type 2 Diabetes Patients. Aging Dis 2019; 10: p. 37-48.
87. Hsu, C.C., et al., Incidence of dementia is increased in type 2 diabetes and reduced by the use of sulfonylureas and metformin. J Alzheimers Dis 2011; 24: p. 485-493.
88. Ng, T.P., et al., Long-term metformin usage and cognitive function among older adults with diabetes. J Alzheimers Dis 2014; 41: p. 61-68.
89. Herath, P.M., et al., The Effect of Diabetes Medication on Cognitive Function: Evidence from the PATH Through Life Study. Biomed Res Int 2016; 2016: p. 7208429.
90. Orkaby, A.R., et al., Metformin vs sulfonylurea use and risk of dementia in US veterans aged >/=65 years with diabetes. Neurology 2017; 89: p. 1877-1885.
91. Bohlken, J., L. Jacob, and K. Kostev, Association Between the Use of Antihyperglycemic Drugs and Dementia Risk: A Case-Control Study. J Alzheimers Dis 2018; 66: p. 725-732.
92. Pomilio, C., et al., Diabetic patients treated with metformin during early stages of Alzheimer’s disease show a better integral performance: data from ADNI study. Geroscience 2022; 44: p. 1791-1805.
93. Newby, D., et al., Comparative effect of metformin versus sulfonylureas with dementia and Parkinson’s disease risk in US patients over 50 with type 2 diabetes mellitus. BMJ Open Diabetes Res Care 2022; 10.
94. Zheng, B., et al., Dementia risk in patients with type 2 diabetes: Comparing metformin with no pharmacological treatment. Alzheimers Dement 2023.
95. Chen, P.C., et al., Metformin Adherence Reduces the Risk of Dementia in Patients With Diabetes: A Population-based Cohort Study. Endocr Pract 2023; 29: p. 247-253.
96. Luchsinger, J.A., et al., Pre-Diabetes, but not Type 2 Diabetes, Is Related to Brain Amyloid in Late Middle-Age. Journal of Alzheimer’s Disease 2020; 75: p. 1241-1252.
97. Ping, F., N. Jiang, and Y. Li, Association between metformin and neurodegenerative diseases of observational studies: systematic review and meta-analysis. BMJ Open Diabetes Res Care 2020; 8.
98. Ye, F., et al., Impact of Insulin Sensitizers on the Incidence of Dementia: A Meta-Analysis. Dement Geriatr Cogn Disord 2016; 41: p. 251-260.
99. Weinstein, G., et al., Association of metformin, sulfonylurea and insulin use with brain structure and function and risk of dementia and Alzheimer’s disease: Pooled analysis from 5 cohorts. PLoS One 2019; 14: p. e0212293.
100. Luo, A., et al., Association Between Metformin and Alzheimer’s Disease: A Systematic Review and Meta-Analysis of Clinical Observational Studies. J Alzheimers Dis 2022; 88: p. 1311-1323.
101. Campbell, J.M., et al., Metformin Use Associated with Reduced Risk of Dementia in Patients with Diabetes: A Systematic Review and Meta-Analysis. J Alzheimers Dis 2018; 65: p. 1225-1236.
102. Campbell, J.M., et al., Metformin and Alzheimer’s disease, dementia and cognitive impairment: a systematic review protocol. JBI Database System Rev Implement Rep 2017; 15: p. 2055-2059.
103. Ji, S., et al., Metformin and the risk of dementia based on an analysis of 396,332 participants. Ther Adv Chronic Dis 2022; 13: p. 20406223221109454.
104. Zhang, Y., et al., Metformin and the risk of neurodegenerative diseases in patients with diabetes: A meta-analysis of population-based cohort studies. Diabet Med 2022; 39: p. e14821.
105. Luchsinger, J.A., et al., Metformin in Amnestic Mild Cognitive Impairment: Results of a Pilot Randomized Placebo Controlled Clinical Trial. J Alzheimers Dis 2016; 51: p. 501-514.
106. Hartman, S.J., et al., The effects of weight loss and metformin on cognition among breast cancer survivors: Evidence from the Reach for Health study. Psychooncology 2019; 28: p. 1640-1646.
107. Koenig, A.M., et al., Effects of the Insulin Sensitizer Metformin in Alzheimer Disease: Pilot Data From a Randomized Placebo-controlled Crossover Study. Alzheimer Dis Assoc Disord 2017; 31: p. 107-113.
108. Lin, Y., et al., Evaluation of Metformin on Cognitive Improvement in Patients With Non-dementia Vascular Cognitive Impairment and Abnormal Glucose Metabolism. Front Aging Neurosci 2018; 10: p. 227.
109. Charpignon, M.L., et al., Causal inference in medical records and complementary systems pharmacology for metformin drug repurposing towards dementia. Nat Commun 2022; 13: p. 7652.
110. Dai, J., et al., Metformin and Dementia Risk: A Systematic Review with Respect to Time Related Biases. J Alzheimers Dis Rep 2022; 6: p. 443-459.
111. Wang, C., et al., Clinical perspectives and concerns of metformin as an anti-aging drug. Aging Med (Milton) 2020; 3: p. 266-275.
112. Markowicz-Piasecka, M., et al., Metformin – a Future Therapy for Neurodegenerative Diseases : Theme: Drug Discovery, Development and Delivery in Alzheimer’s Disease Guest Editor: Davide Brambilla. Pharm Res 2017; 34: p. 2614-2627.
© Serdi 2023