L. Jönsson, A. Wimo
ection for Neurogeriatrics, Department for Neurobiology, Care Sciences and Society, Karolinska Institute, Sweden.
Corresponding Author: Anders Wimo, Section for Neurogeriatrics, Department for Neurobiology, Care Sciences and Society, Karolinska Institute, Sweden, anders.wimo@ki.se
J Prev Alz Dis 2023;3(10):349-352
Published online June 13, 2023, http://dx.doi.org/10.14283/jpad.2023.76
Introduction
After decades of unsuccessful trials, halted development programs and billions of dollars invested in developing disease-modifying therapies for Alzheimer’s disease (AD) (1), there is renewed hope of treatments that impact the progression of this devastating illness. Two antibodies targeting amyloid-β, lecanemab and donanemab, have shown clinical efficacy (in phase-3 trials) on the progression of AD in early AD, defined as mild cognitive impairment (MCI) due to AD or mild AD dementia (2, 3). Both treatments have demonstrated effects on cognitive and functional endpoints, as well as biomarkers, over 18 months of treatment. Lecanemab has received fast-track regulatory approval in the United States, while regulatory review by the European Medicines Agency (EMA) is still ongoing.
Questions have been raised regarding the clinical meaningfulness of the effect sizes, the long-term effects, the risk of adverse events, primarily amyloid-related imaging abnormalities (ARIA-E and ARIA-H) and the feasibility of administering and monitoring the treatment in a routine care setting (4-8). In addition, concern has been raised about the affordability of these treatments, and whether they will provide good value for money for health care systems (9, 10).
Pharmaceutical pricing and reimbursement
Pharmaceuticals have increased in importance as a production factor in health care systems over the past decades, both in terms of contributions to improved health and in terms of the share of overall health care costs. In OECD countries, pharmaceutical expenditures as a share of GDP have increased from 0.9% in 1990 to 1.4% in 20211. As a response, many countries have established institutions and processes aimed at securing value for money of drug spending as well as capping increases in pharmaceutical expenditures, such as health technology assessment bodies and pricing and reimbursement agencies2. Decisions to restrict access to a treatment on the basis of cost or cost-effectiveness can be politically difficult, underscoring the need for independent agencies that have a responsibility towards all patients, not only the ones who might benefit from a novel therapy. The alternatives for spending health care resources far exceed available budgets, which means a decision to reimburse a treatment invariably leads to some other service being negatively affected or discontinued, unless budgets are simultaneously increased. This crowding-out effect is however spread over the entire health care system and almost never explicitly referred to in reimbursement decision making, thus those who benefit from a decision not to reimburse a cost-ineffective drug are not easily identified.
Attempting to regulate pharmaceutical prices is a complex problem with several inherent goal conflicts. Besides restricting increases in drug costs, health care systems wish to have timely access to effective treatments, both today and in the future. If drug prices are restricted this may adversely affect incentives for investing in future development programs. Pricing and reimbursement decisions must be based on evidence, ideally from studies that include unselected patients under conditions similar to those under which the treatment will be used in routine care and follow patients long enough to observe all relevant outcomes, over time. Collecting such evidence is costly, and requiring high evidentiary standards for pricing and reimbursement decisions can lead to further increases in drug costs3. Of note, smaller countries have limited ability to influence how studies are designed in multinational drug development programs.
1. Calculated as an average for countries with available data: Australia, Austria, Belgium, Canada, Czech Republic Denmark, Finland, France, Germany, Greece, Iceland, Ireland, Italy, Japan, Korea, Luxembourg, Netherlands, Norway, Portugal, Spain, Sweden, United Kingdom and United States. 2. A notable exception is the United States, where so far health economic deliberations play a limited role in pharmaceutical pricing and reimbursement; 3. Even if the decision is not to reimburse the drug, the cost of generating the evidence would have to be borne by other, successful, development projects.
The role of cost-effectiveness analysis
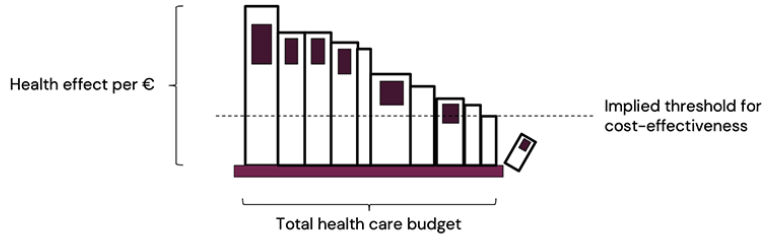
Cost-effectiveness is not the same as cost savings. There is always a willingness to pay for a treatment that gives good value for money. This situation has been compared to a bookshelf, where each book corresponds; to a health care program (11). The height of the book is proportional to the cost-effectiveness of the program, i.e. how much health is generated for each invested euro or dollar, and the width of the book signifies the budget impact of the program (the number of patients times the cost per patient per year). The bookshelf is fully stacked from end to end, and ordered by height such that only the most cost-effective programs (highest books) remain implemented on the bookshelf. The shortest book on the shelf represents the program that ‘just made it’ in terms of cost-effectiveness, and thus implicitly the threshold cost-effectiveness ratio that any new program needs to beat in order to be implemented. This ensures that the health effect from the available budget is maximized; no other combination of health care programs will lead to higher health effects for the available budget.
A new program, e.g. a disease-modifying therapy for Alzheimer’s disease, will necessarily push other books off the shelf – in particular since this particular program will have a very large footprint. In fact, it may replace several programs, each with better cost-effectiveness than the previous. Thus a program with a large budget impact may need to demonstrate stronger evidence for good cost-effectiveness than a smaller program.
The evidence for cost-effectiveness of disease-modifying therapies for Alzheimer’s disease is now at its lowest point, having only data from clinical trials but no experience from how these drugs will perform under routine care conditions and what the long-term outcomes will be. Yet health care systems will very soon need to make important decisions regarding if and how to start implementing these therapies. Health economic modelling is frequently used as an attempt to bridge this gap by combining trial data with epidemiological projections and information from cost-of-illness and quality-of-life studies, projecting the potential costs and health benefits into the future.
Assessing the value and ‘fair price’ of amyloid-targeting therapies
At present, two economic models of lecanemab have been published evaluating the cost-effectiveness based on the announced pricing in the United States, one by the company marketing the drug (12), and one by the Institute of Clinical and Economic Review (ICER) (13). Both models, though based on different structures and assumptions, project modest or no cost savings (in non-treatment-related costs) but substantial health gains in terms of quality adjusted life years (QALYs) gained, as well as overall survival. The company-sponsored model extrapolated the 18 months gap between the slopes of treated and non-treated people to a simulated life-long period, resulting in 2.13 AD free-years based on 3.91 years of treatment (12). Survival effects have a dramatic effect on both the health gains and expected cost impact of disease-modifying treatment. As disease modification is assumed to lead to a reduction of mortality (as patients remain for longer in less severe disease stages where mortality is lower), this generates life-years and QALYs gained but also offsets cost savings from reduced disease progression since patients incur higher costs while living longer. It should be noted that no survival benefit has been demonstrated for any disease-modifying therapy, so this effect is entirely hypothetical at present. Further, it should be underscored that the models do not project net reductions in costs for care for AD patients, which is at odds with the expectation that slowing disease progression might reduce the demand on long-term care services.
The suggested prices for adecanumab and lecanumab have caused a great debate, mainly because of the potential great budget impact of DMTs (9), not withstanding the added medical costs required for treatment efficacy and safety monitoring. Treatment with antibodies are often expensive, but a high price per se is not the problem. However, early AD is a highly prevalent disorder affecting millions of people worldwide (14); hence offering treatment to all patients within the target indication may lead to unsustainable budget impact. The European Alzheimer´s Disease Consortium (EADC) has estimated that if the U.S. list price is applied in the EU, this single drug would consume about half the budget for all pharmaceuticals in Europe (15).
Managing uncertainty
Given the considerable uncertainty around the long-term clinical and economic outcomes, decision makers may be reluctant to grant wider access to the treatments (i.e. unconditional approval and reimbursement) until more evidence is available. For instance, the California Technology Assessment Forum (CTAF) panel unanimously voted that lecanemab at its current price provides low long-term value for money, and noted concerns about the uncertain durability of benefit of lecanemab treatment given the lack of long-term data (13). Further the Center for Medicare and Medicaid Services (CMS) has ruled that no disease-modifying agent for AD will be reimbursed outside of clinical studies based only on short-term clinical trial data (16). However, waiting until long-term follow-up data is available would mean severely restricting access to treatments with potentially important health benefits for many years – and the pace of evidence generation would be considerably slower compared to if these treatments were widely used and could be studied in large-scale follow-up studies.
It is clear that conventional pricing and reimbursement models will not succeed in resolving these issues. A potential path out of this apparent catch-22 would be to spread risks over time and to share risks between the manufacturer and health care payers through the use of contracting mechanisms that allocate payment depending on follow-up studies reaching predefined endpoints (outcome-based payment) (17). This could immediately lower the thresholds for introducing the treatments, while maintaining appropriate incentive structures for innovators and manufacturers. Managed entry agreements, a broader term encompassing financial as well as performance-based contracts, have been used in other disease areas and have become more common over time (18), though the implementation of outcome-based agreements has limited to a handful of countries and regions . The obvious drawback of such programs is the cost and complexity of implementation, requiring long follow-up studies as well as legally binding and enforceable contracts that span over many years (and mandate periods). There are today very few established dementia registries worldwide, and those that exist are often struggling with maintaining continuity of follow-up and coverage levels. Strengthening registry infrastructure would enable outcome-based reimbursement mechanisms to be implemented without prohibitive costs to any single company or health care provider.
Conclusions and recommendations
We believe that a reasonable approach to reimbursement for amyloid-targeting therapies would allow patients to access treatment based on objective criteria related to the ability to benefit from treatment, and the balance between expected benefits and the risk of adverse events. At the same time, the reimbursed price levels must factor in the high degree of uncertainty regarding long-term clinical and economic benefits. It is unreasonable to assign prices as if the evidence on long-term outcomes was already available and conclusive. The costs of identifying patients for treatment (e.g. by expanding amyloid biomarker utilization), as well as for the administration and monitoring of treatment should all be considered as part of the intervention cost when assessing the cost-effectiveness of amyloid-targeting therapies. Health care systems have an interest in generating this evidence, and should make necessary investments in infrastructure for diagnosis and treatment but importantly also for systematic follow-up and assessment of long-term outcomes and safety.
The global pandemic of Alzheimer´s Disease (AD) and other dementias, with 55 million people affected in 2019 and forecasted to about 140 million in 2050, combined with the huge economic impact – 1.3 trillion US$ in 2019 (19) – highlights the enormous challenges for any society. If costs are added for prodromal and preclinical stages of AD, the situation is even more dramatic (14). Amyloid-targeting therapies offer a first step towards addressing the challenge this disease poses. How they are utilized in routine care will have important implications, not only for the patients and their loved ones, but also for future investments in research and drug development. The development of treatments that reduce the rate of progression by almost a third is a great achievement, and if used wisely they may lead to improvements for patients and caregivers that would not have been possible before this innovation. However we are still far from being able to arrest or reverse the disease course. We need even better DMTs as well as a focus on combined approaches, including optimal diagnostic pathways and non-pharmacological prevention strategies. Getting the reimbursement of the current DMTs right will be an important step in this direction.
Conflict of interest: Dr. Wimo reports license holder of the RUD instrument. Dr. Jönsson reports grants from Novo Nordisk, outside the submitted work; In addition, Dr. Jönsson has a patent RUD instrument licensed to European Health Economics AB.
References
1. Cummings, J. L., Goldman, D. P., Simmons-Stern, N. R., andPonton, E. The costs of developing treatments for Alzheimer’s disease: A retrospective exploration Alzheimers Dement 2022;18, 469-477 10.1002/alz.12450
2. van Dyck, C. H., Swanson, C. J., Aisen, P., Bateman, R. J., Chen, C., Gee, M. et al. Lecanemab in Early Alzheimer’s Disease N Engl J Med 2023;388, 9-21 10.1056/NEJMoa2212948
3. Lilly (2023) Lilly’s Donanemab Significantly Slowed Cognitive and Functional Decline in Phase 3 Study of Early Alzheimer’s Disease Lilly, ed. Lilly, 3
4. Frölich, L., and Jessen, F. Lecanemab: Appropriate Use Recommendations — A Commentary from a European Perspective. J Prev Alz Dis 2023;3(10):357-358. dx.doi.org/10.14283/jpad.2023.44
5. Mahase, E. Lecanemab trial finds slight slowing of cognitive decline, but clinical benefits are uncertain BMJ 2022;379, o2912 10.1136/bmj.o2912
6. McDade, E., Cummings, J. L., Dhadda, S., Swanson, C. J., Reyderman, L., Kanekiyo, M. et al. Lecanemab in patients with early Alzheimer’s disease: detailed results on biomarker, cognitive, and clinical effects from the randomized and open-label extension of the phase 2 proof-of-concept study Alzheimers Res Ther 2022;14, 191 10.1186/s13195-022-01124-2
7. The, L. Lecanemab for Alzheimer’s disease: tempering hype and hope Lancet 2022;400, 1899 10.1016/S0140-6736(22)02480-1
8. Walsh, S., Merrick, R., Richard, E., Nurock, S., and Brayne, C. Lecanemab for Alzheimer’s disease BMJ 2022;379, o3010 10.1136/bmj.o3010
9. Wimo, A., Jönsson, L., Johansson, G., and Winblad, B. (2023) Lecanemab: The Price of a Breakthrough touchREVIEWS in Neurology 19, https://touchneurology.com/alzheimers-disease-dementia/journal-articles/lecanemab-the-price-of-a-breakthrough/#article-information
10. Jönsson, L., Wimo, A., Handels, R., Johansson, G., Boada, M., Engelborghs, S. et al. (2023) The affordability of lecanemab, an amyloid-targeting therapy for Alzheimer’s Disease: an EADC viewpoint The Lancet Regional Health – Europe (in press),
11. Culyer, A. J. Cost-effectiveness thresholds in health care: a bookshelf guide to their meaning and use Health Econ Policy Law 2016;11, 415-432 10.1017/S1744133116000049
12. Tahami Monfared, A. A., Tafazzoli, A., Chavan, A., Ye, W., and Zhang, Q. The Potential Economic Value of Lecanemab in Patients with Early Alzheimer’s Disease Using Simulation Modeling Neurol Ther 2022;11, 1285-1307 10.1007/s40120-022-00373-5
13. ICER (2023) ICER Publishes Evidence Report on Lecanemab for Alzheimer’s Disease ICER, ed. Institute for clinical and economic review (ICER),
14. Gustavsson, A., Norton, N., Fast, T., Frolich, L., Georges, J., Holzapfel, D. et al. (2022) Global estimates on the number of persons across the Alzheimer’s disease continuum Alzheimers Dement 10.1002/alz.12694
15. Jönsson, L., Wimo, A., Handels, R., Johansson, G., Boada, M., Engelborghs, S. et al. (2023) The affordability of lecanemab, an amyloid-targeting therapy for Alzheimer’s Disease: an EADC-EC viewpoint The Lancet Regional Health – Europe 29,
16. CMS (2022) CMS Finalizes Medicare Coverage Policy for Monoclonal Antibodies Directed Against Amyloid for the Treatment of Alzheimer’s Disease Center for Medicare and Medicaid Services (CMS),
17. Hung, A., Hamilton Lopez, M., Schneider, M., andMcClellan, M. (2020) Addressing Challenges in Payment and Access to Treatments for Early-Stage Alzheimer’s Disease Duke Margolis Center for Health Policy,
18. Ciulla, M., Marinelli, L., Di Biase, G., Cacciatore, I., Santoleri, F., Costantini, A. et al. Healthcare Systems across Europe and the US: The Managed Entry Agreements Experience Healthcare (Basel) 2023;11, 10.3390/healthcare11030447
19. Wimo, A., Seeher, K., Cataldi, R., Cyhlarova, E., Dielemann, J. L., Frisell, O. et al. (2023) The worldwide costs of dementia in 2019 Alzheimers Dement 10.1002/alz.12901
© Serdi 2023