M.T. Ashford1,2,*, D. Zhu3, J. Bride4, E. McLean4,5, A. Aaronson1,6, C. Conti1,2, C. Cypress7, P. Griffin7, R. Ross1,2,7, T. Duncan7, X. Deng3, A. Ulbricht1,6, J. Fockler1,6, M.R. Camacho1,2, D. Flenniken1,2, D. Truran1,2, S.R. Mackin1,8, C. Hill9, M.W. Weiner1,2,6,12,13, D. Byrd10, R.W. Turner II4, H. Cham3, M. Rivera Mindt11, R.L. Nosheny1,8
1. VA Advanced Imaging Research Center, San Francisco Veteran’s Administration Medical Center, San Francisco, CA, USA; 2. Northern California Institute for Research and Education (NCIRE), Department of Veterans Affairs Medical Center – San Francisco, USA; 3. Department of Psychology, Fordham University, USA;
4. School of Medicine and Health Sciences, Department of Clinical Research and Leadership, The George Washington University, USA; 5. Department of Psychology, American University, USA; 6. Department Of Radiology and Biomedical Imaging, University of California San Francisco – San Francisco, USA; 7. CEDAR Community Scientific Partnership Board, USA; 8. Department of Psychiatry, University of California San Francisco – San Francisco, USA, UCSF, Department of Neurology, San Francisco, CA, USA; 9. Alzheimer’s Association, Chicago, IL, USA; 10. CUNY, Queens College, Queens, NY, USA; 11. Psychology & Latin American Latino Studies Institute, Fordham University, Joint Appointment in Neurology, Icahn School of Medicine at Mount Sinai – New York, USA; 12. Department of Neurology, University of California San Francisco, San Francisco, CA, USA; 13. Department of Medicine, University of California San Francisco, San Francisco, CA, USA
Corresponding Author: Miriam Ashford, 4150 Clement St, San Francisco, CA 94121, Email: Miriam.ashford@ucsf.edu, Phone: (415) 750-6954, Fax number: (415) 750-9358
J Prev Alz Dis 2023;3(10):551-561
Published online March 14, 2023, http://dx.doi.org/10.14283/jpad.2023.25
Abstract
BACKGROUND: Failure of Alzheimer’s disease and related diseases (ADRD) research studies to include and engage Black participants is a major issue, which limits the impact and generalizability of research findings. Little is known about participation of Black adults in online ADRD-related research registries.
OBJECTIVES: As part of the Community Engaged Digital Alzheimer’s Research (CEDAR) Study, this study aims to increase our understanding of facilitators and barriers of Black adults to participating in ADRD-related online registries, as well as to understand their preferences for communication channels.
DESIGN, SETTING, PARTICIPANTS, MEASUREMENTS: We invited all Black participants enrolled in the Brain Health Registry (BHR) to complete a cross-sectional online survey. The survey consisted of rating scales and open-text questions asking about their attitudes towards brain health research, reasons for joining and continuing to participate in BHR, difficulties with participating, and preferences for modes of contact and website usage.
RESULTS: Of all invited Black BHR participants (N=3,636), 198 (5.5%) completed the survey. The mean age was 58.4 (SD=11.3), mean years of education were 16.3 (SD=2.4), and 85.5% identified as female. Reported facilitators for joining and continuing to participate in BHR were personal interest (e.g., learning more about own brain health) and altruism (e.g., helping research). Among additional registry features which could encourage return, receiving feedback or scores about BHR tasks was rated the highest. Of those who found BHR participation difficult (21%), the most frequent reason was time burden. The most preferred way of receiving study information was via email. Participants reported that the websites that they used the most were YouTube and Facebook.
DISCUSSION: The results of our study can inform the development of culturally-responsive registry features and engagement efforts to improve inclusion and participation of Black adults in online ADRD research. Providing participants with feedback about their registry performance and reducing the number of registry tasks are among the recommended strategies.
Key words: Brain Health Registry, facilitators, barriers, african americans, black americans, Alzheimer’s disease, dementia, brain health, health equity, health disparities, survey.
Introduction
Alzheimer’s disease and related dementias (AD/ADRD) are a large and growing public health threat (1). The incidence of AD is disproportionately high in Black/African American (hereafter referred to as Black) communities and they may have more risk factors (e.g., heart health issues) compared to the non-Latinx White population (2, 3). The incidence of AD and risk factors are often attributed to a combination of sociocultural (e.g., discrimination, racism, access to quality healthcare and education) and structural determinants of health (e.g., government process, policies) (4) and vascular, body mass index, and genetic factors (3, 5-10). Furthermore, research studies suggest that AD course, symptom severity, and medication response are different in Black adults versus non-Latinx White adults (3, 6). Black individuals also experience earlier age of onset, less APOE ε4 allele association with AD, and more severe cognitive impairment (3). Yet, our understanding of AD health disparities in Black individuals remains obscured by lack of data and inconsistencies in findings (11). One contributing factor is the chronic under-inclusion of Black individuals in AD/ADRD related research studies (12-16). Under-inclusion limits the reach of dementia science, reduces the generalizability and validity of findings, and limits our ability to understand health disparities.
Previous literature has documented structural and systemic barriers, as well as historical events (e.g., Tuskegee study) which contribute to the failure of research studies to include participants from Black communities. For example, common barriers include a failure of research studies to provide education about AD and research, transportation to studies, and appropriate compensation for participation, as well as the failure of researchers to gain the trust of Black communities (17-21). A thorough understanding of the structural, systemic, and individual barriers that lead to failure to include Black community members is needed to move the field forward.
The Brain Health Registry (BHR) (22) is an AD/ADRD-related public online registry (N>95,000) which longitudinally assesses cognition, health, and function and refers participants to other in-clinic and online studies. However, like in-clinic studies and trials, many online registries, including BHR, under-include and underrepresent Black participants (22-24) when compared with the US Census data (22, 25). Further, BHR has so far failed to substantively engage Black participants, evidenced by lower completion rates of study tasks, lower rates of return for follow-up study visits, and lower levels of participation in additional genetic and biomarker research compared to non-Latinx White BHR participants (26). To begin to address BHR’s failure to engage Black participants, the CEDAR: Community Engaged Digital Alzheimer’s Research project was founded as a sub-study of the BHR. CEDAR aims to increase the engagement and retention of currently enrolled BHR participants who self-identify as Black. As part of the CEDAR study, we conducted a cross-sectional mixed methods online survey among currently enrolled Black BHR participants to broaden our understanding of their facilitators and barriers to BHR participation, as well as preferences for engagement and communication channels. Herein we provide a quantitative and qualitative summary of the survey responses, which can be utilized to develop culturally-informed BHR engagement strategies for Black participants in our study and more broadly in the AD/ADRD research field.
Methods
The survey data presented in this paper was collected as part of the CEDAR: Community Engaged Digital Alzheimer’s Research project, a sub-study of the BHR. The CEDAR project aims to increase the inclusion, engagement, and retention of currently enrolled BHR participants who self-identify as Black. As a first step of this study, Black BHR participants were invited to complete a cross-sectional online barriers and facilitators survey, which will be used to inform development of engagement and retention strategies. A description of the larger CEDAR project and results will be reported in a separate manuscript.
Brain Health Registry
The BHR is a publicly available voluntary online ADRD-related research registry to recruit, screen, and longitudinally assess adults (aged 18 and older), as well as to refer participants for cognitive- and aging-related research (22, 27, 28). BHR participants are recruited from the general public using many methods, for example, digital advertising, cross-promotion with other studies and registries, and earned media. The registry includes an online consent, self-report questionnaires (e.g., sociodemographic, health, cognitive, and lifestyle), self-administered neuropsychological tests, and study partner enrollment and questionnaires. Participants are invited to complete the online neuropsychological tests and questionnaires every 6 months.
Overall approach
All BHR participants self-identifying as Black who agreed to be contacted about future research opportunities were invited to join the CEDAR study, including the survey, via a series of culturally-informed emails. Only participants enrolled in the CEDAR study were presented with the opportunity to complete the survey. This voluntary cross-sectional survey was hosted by Qualtrics and was available from August 2021 to April 2022 and available in English only. The UCSF Institutional Review Board approved the survey. The survey took approximately 10-15 minutes to complete and was suitable for completion via mobile devices and laptops/computers. Before the launch, the study team tested the survey’s technical functionality. There was a financial incentive for completing this survey and/or BHR remote assessments. Reminder emails with culturally-informed messaging were sent to all invited participants who did not sign the study consent (day 8, 15, and 30 after original invitation email), as well as to those who signed the consent, but who did not start the survey within 3 days of signing. Reminders to complete the survey were implemented in March 2022. Of 3,641 invited participants, eleven (0.3%) started the survey two or more times. Only the survey entry with the higher number of answered survey questions was used.
Measures
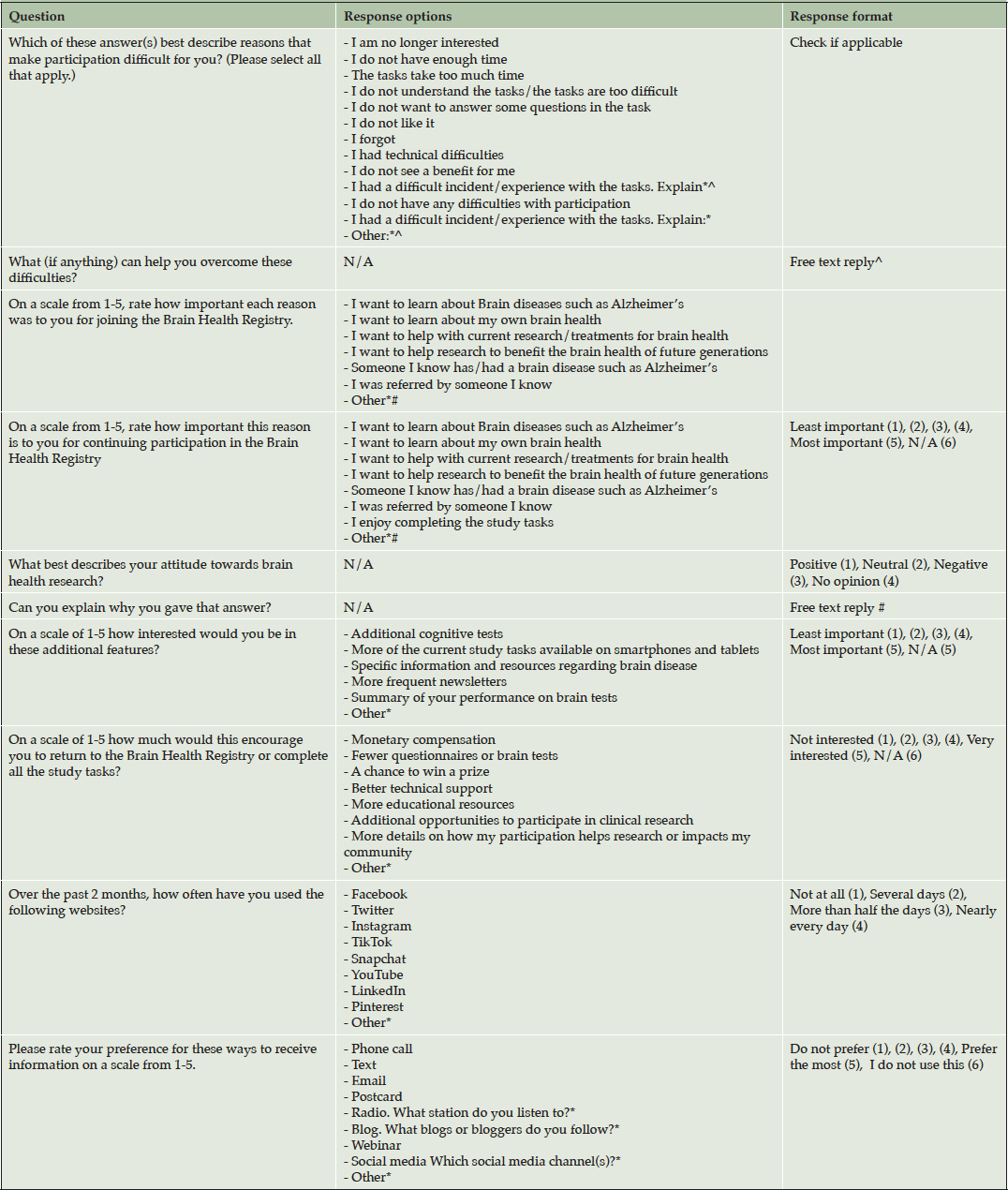
Questions were developed by reviewing the literature regarding research engagement and were discussed among the research team to establish content validity. This online survey consisted of 11 questions, including one multiple choice question allowing multiple responses, seven matrix tables in which respondents rated statements/answer options on scales, and six questions with free-text responses. All questions were optional. Please see Table 1 for all survey questions in the order in which they were administered, as well as the available answer options and format.
Respondent sociodemographic data
Participants self-report the following within the BHR online portal: race (Asian, Black/African American, White, Native American, Pacific Islander, Other, decline to state); ethnicity (Latino, non-Latino, declined to state); age (continuous); gender (male, female, other, unknown); educational attainment (grammar school, high school, some college, two-year degree, four-year degree, Master’s degree, doctoral degree, professional degree); endorsement of subjective memory concern (“Are you concerned that you have a memory problem?”); and family history of AD/dementia (“Do you have any biological parents, full siblings, or biological children who have been diagnosed with Alzheimer’s Disease?”). The variable educational attainment was converted into a continuous variable ranging from 6-20 years. A new race variable was created with the categories “Black only”, “Black and other races”, and “declined to state”. If information was missing for the variables gender, ethnicity, and race, we also added a category “unknown”.
Statistical Analysis
Descriptive statistics for demographic variables including percentages, n, means, and standard deviations (SD) were calculated for all invited participants. For continuous variables such as age and years of education, independent sample t-tests were conducted to compare means between those who did not respond to the survey and those who responded. Cohen’s d was reported as effect size. For categorical variables such as gender and race, chi-square tests for association were used if ≤ 20% of expected cell counts were less than five and Cramer’s V was reported as effect size. Fisher’s exact tests were used if > 20% of expected cell counts were less than five. Both completed and partial responses were analyzed. Frequencies for each option were provided. If a question had a scale of more than four categories, we regarded it as continuous variable, calculated the mean and standard deviations. We also conducted pairwise comparison using Bonferroni correction to test if the mean score difference was statistically significantly different between the different question statements. All statistical analyses were done in R (4.2.0) (29) and using the package “psych”.
Qualitative Analysis
The data of the free-text responses was analyzed using an open-coding approach and thematic analysis (30). As the associated questions were optional, blank responses were skipped. First, to become familiar with the data, two members of the research team who were not involved in the design and administration of the survey read the free-text responses at least two times and drafted initial categories. Second, they met to openly code, assess the categories, and confirm themes. The research team determined that the six survey questions with free-text responses could be divided between two overarching research interests: “Research participation” and “Barriers to research participation”. See Table 1 for information about which questions were included in each of the two interests. Classifying the questions allowed the research team to develop themes and categories separately for each interest.
Note. *=Includes free text reply option; #=questions analyzed in qualitative analysis of “Research Participation; ^=questions analyzed in qualitative analysis of “Barriers to Research Participation”
Results
Survey response
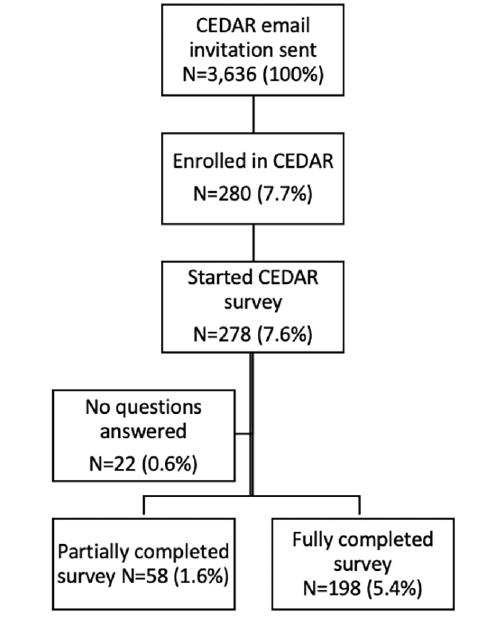
A total of 3,641 Black BHR participants were invited to enroll in CEDAR and of those 280 (7.7%) enrolled and 278 (7.6%) started the survey. Of those enrolled in CEDAR and presented with the survey, 256 (91.4%) answered the survey. Figure 1 presents the attrition from invitation to completed survey, including information about partial responses. There were 22 BHR participants who started the survey, but did not answer any questions, 58 partial responses, and 198 complete responses. Of all who started the survey, 256 (92.1%) had at least partial data.
Sample characteristics
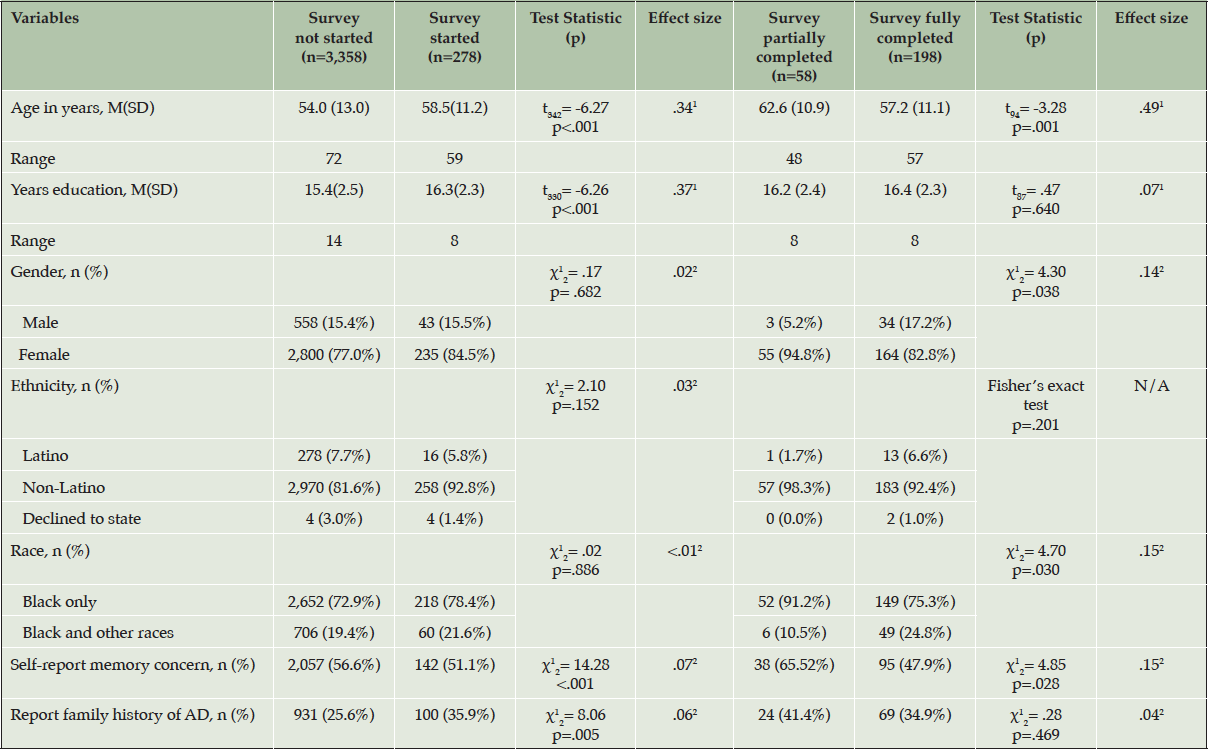
Detailed information about the characteristics of the survey respondents is given in Table 2. Compared to those who did not start the survey, those who started reported that they were significantly older (t342=6.28, p<.001, Cohen’s d=.34) with more years of education (t330=6.26, p<.001, Cohen’s d=.37), were more likely to report a family history of AD, (χ12=8.06, p=.005, Cramer’s V=.05), and less likely to report a memory concern, (χ12=14.28, p<.001, Cramer’s V=.06). All results following this participant characteristic section use data of respondents with at least partial responses (N=256). Respondents with at least partial data (this includes respondents with partial and complete data) were on average 58.44 years old (SD=11.27), 16.31 years of education (SD=2.35), and most identified as female (85.5%, n=219) and as Black/ only (79.7%, n=201). Fourteen (0.5%) self-identified as Latinx/o/a. A total of 93 (36.3%) participants self-reported a family history of AD and 133 (52.0%) self-reported memory concerns. Table 2 also compares the demographic characteristics of those who partially completed the survey and those who fully completed it.
Note. 1= Cohen’s d, 2= Cramer’s V.
Research Participation
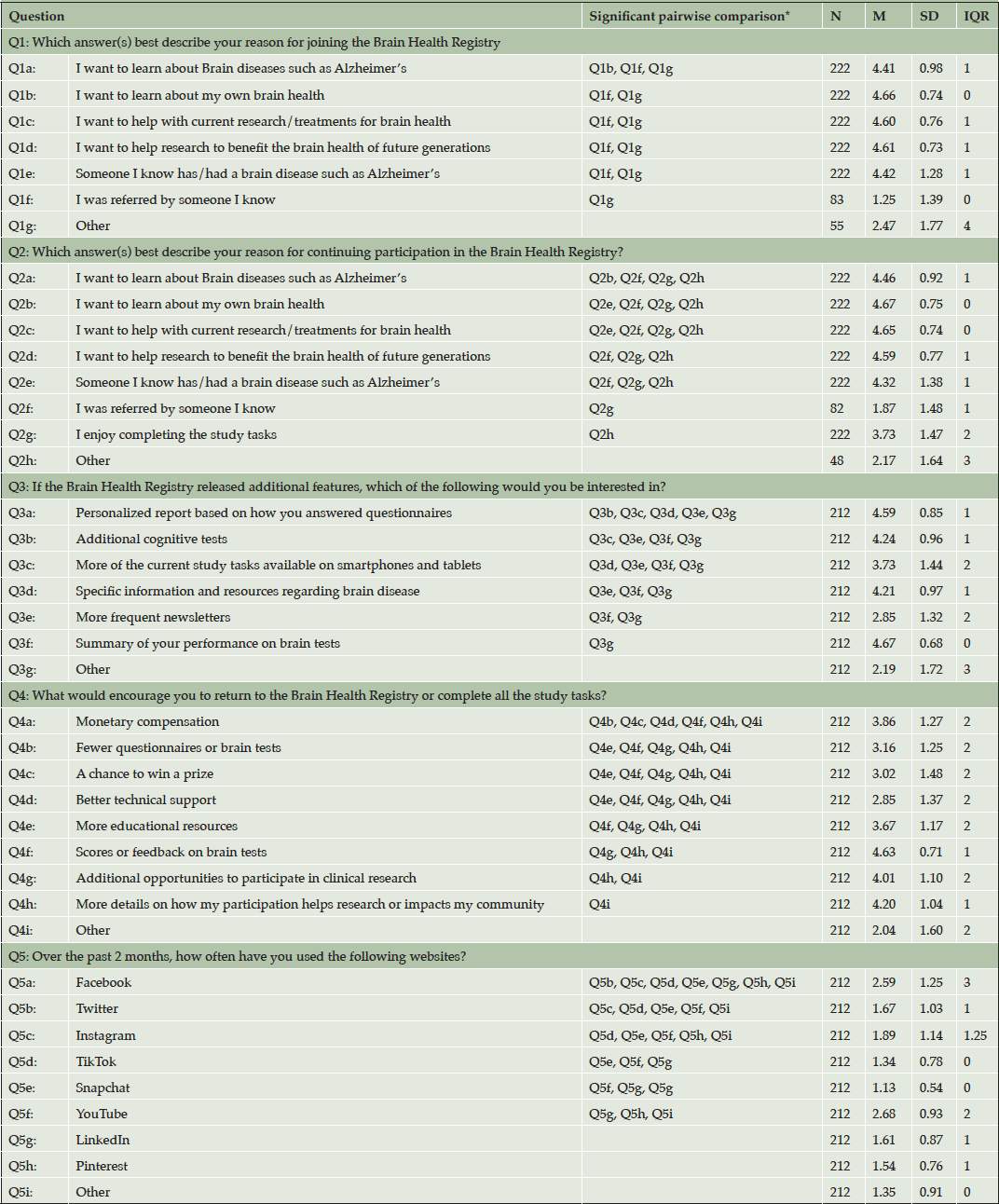
Table 3 provides summary statistics and information about pairwise comparison for all survey questions.
Note. *Significant pairwise comparisons with Bonferroni correction. For example, for Q1a, this demonstrates that the mean of the response option Q1a (I want to learn about Brain diseases such as Alzheimer’s) is significantly different from the mean of the response options Q1b (I want to learn about my own brain health), Q1f (I was referred by someone I know), and Q1g (Other). The scale for Questions 1 through 4 ranges from “1”-“5.” The scale for Question 5 ranges from “1”–“4.”
Attitudes towards brain health research
Most respondents (80.5%, n=206) described their attitude towards brain health research as positive.
Reasons for joining BHR
Participants were asked to rate the importance of different reasons for joining the BHR. The two highest rated statements were: “I want to learn about my own brain health” (M=4.66, SD=.74) and “I want to help research to benefit the brain health of future generations” (M=4.61, SD=.73). There was no significant difference between means of the two highest rated statements (p = .10, Cohen’s d= .21). The statement with the lowest importance rating was “I was referred by someone I know” (M=1.25, SD=1.39) and compared to the two highest rated statements, the statement received a significantly lower rating of importance.
Reasons for continuing to participate in BHR
When asked about the importance of different reasons for continuing to participate in BHR, the following two statements received the highest ratings: “I want to learn about my own brain health” (M=4.67, SD=.75), “I want to help with current research/treatments for brain health” (M=4.65, SD=.74). No significant mean difference was found between the two highest rated statements. The statement which received the lowest rating of importance was “I was referred by someone I know” (M=1.87, SD=1.48) and pairwise comparisons showed that this mean was significantly lower than the means of the two highest rated statements.
Research participation qualitative results
Participants expressed their interest in research participation across five themes: relationships, identity, personal knowledge, altruism, and diversity. See Table 4 for themes, subcategories of themes, as well as counts and quotes for each subcategory.
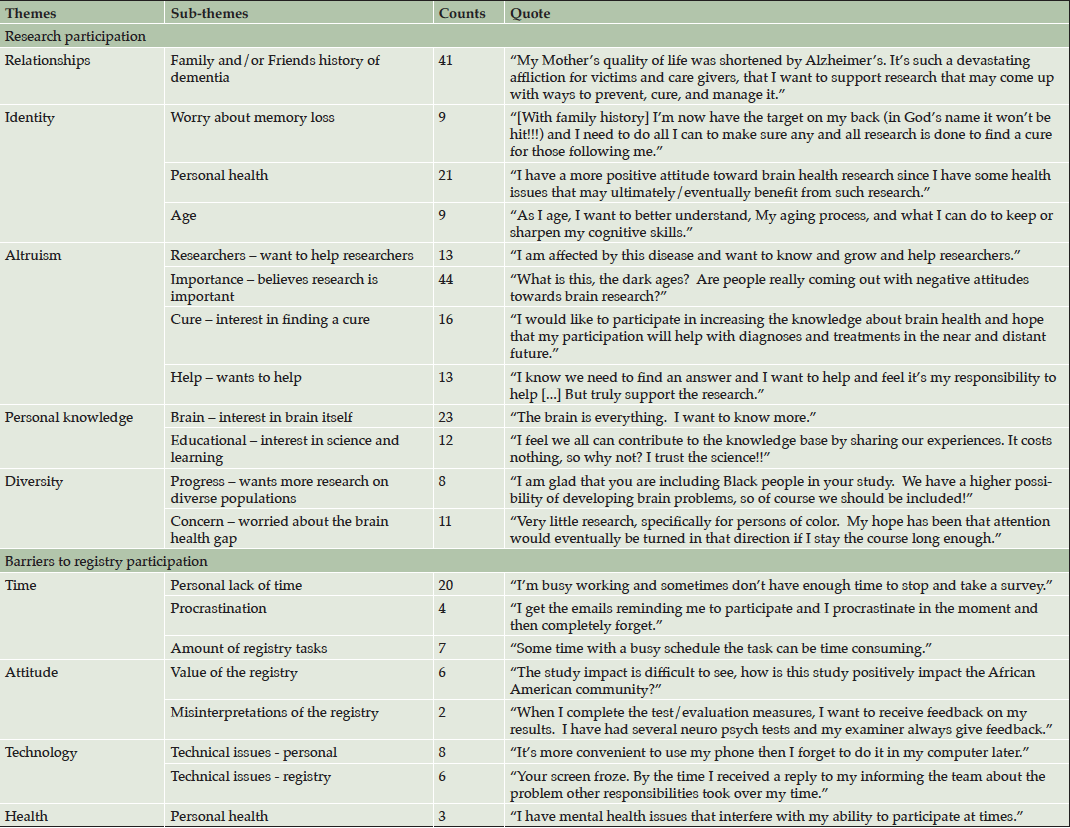
Table 4. Themes, sub-themes, counts and quotes for research participation and barriers to registry participation
Relationships. Most participants stated an interest in participating in brain health research because they had a family history of AD or dementia. Those with personal experience interacting with afflicted loved ones wanted to participate to improve their outlook and the outlook of their friends and family.
Identity. For the identity theme, three sub-categories emerged: Worry about memory loss, personal health, and age. With regard to “worry about memory loss,” many participants were mindful of their own brain health and some admitted participating in BHR because they are worried about memory loss in their future. Participants were aware of their age and the effects of getting older on their personal health. Therefore, they were interested in maintenance of their current brain health and prevention of future issues.
Altruism. For altruism, four sub-categories were identified: 1) researchers – want to help researchers; 2) importance – believes research is important; 3) cure – interest in finding a cure; 4) help – wants to help. The largest subcategory of responses was from participants who highlighted the importance of research. These responses included statements about positive attitudes or beliefs that brain health research is important. Common responses also discussed prevention of brain health diseases and several responses specifically mentioned treatment because of hope that researchers discover a cure.
Personal knowledge. For this theme, two sub-categories were identified: 1) brain – interest in brain itself and 2) educational – interest in science and learning. Several participants were eager to participate due their interest in the brain and related science. Several participants also saw participating in BHR as an opportunity to learn.
Diversity. Respondents cited interest in increasing the diversity within the present study and AD research in general and also worries about the brain health research gap for diverse populations.
Reasons which make participation in BHR difficult
The majority 202 (78.9%) answered “I do not have any difficulties with participation.” Among those who reported difficulties (n=54), the most common reasons were “I don’t have enough time” (n=19, 35.2%) and “The tasks take too much time” (n=19, 35.2%).
Barriers to research participation qualitative results
When asked to describe why participation in the survey was difficult, participants’ explanations followed four themes: time, attitude, technology, and health. See Table 3 for themes, subcategories of themes, as well as counts and quotes for each subcategory.
Time. The majority of barriers indicated by participants fell into the category of time. Among the participants who indicated time as a barrier, lack of personal time was the most frequent, followed by participants reporting that the amount of registry tasks is burdensome. Few, but some participants reported procrastination, or putting tasks off until later, as a barrier to participating in the registry.
Attitude. Several barriers to participation were associated with participant’s perceptions of the registry itself. Perceiving little to no value of the registry was reported most frequently. Additionally, some participants seemed to not understand the purpose of the registry.
Technology. Participants reported both personal technical issues (e.g., inconvenience of having to use a computer), as well as technical issues with the registry website.
Health. Some participants reported that personal health was a barrier to participation. However, health was the least frequently cited barrier of participation.
Additional BHR features which encourage return or completion of study tasks
In terms of additional BHR features suggested in the survey, participants were most interested in “Summary of your performance on brain tests” (M=4.67, SD=.68), followed by “Personalized report based on how you answered questionnaires” (M=4.59, SD=.85). Post-hoc comparisons found no difference between the means of the two highest rated statements. The statement with the lowest mean was “More frequent newsletters” (M=2.85, SD=1.32), which was significantly lower than the means of the two highest rated statements.
Respondents were also asked to indicate the importance of the factors that encouraged them to return to the BHR or to complete all the study tasks. Participants were most encouraged by “Scores or feedback on brain tests” (M=4.63, SD=.71), followed by “More details on how my participation helps research or impacts my community” (M=4.20, SD=1.04). Pairwise comparison revealed that the mean rating of “Scores or feedback on brain tests” was significantly higher than the mean rating of “More details on how my participation helps research or impacts my community.” The statement with the lowest mean rating was “Better technical support” (M=2.85, SD=1.37), which was also significantly lower rated than the two highest rated statements.
Preference for ways of receiving information
In terms of preferred ways of receiving information, respondents preferred email most (M=4.64, SD=.81), followed by text (M=3.75, SD=1.46). The least preferred ways were phone calls (M=2.35, SD=1.54) and blog posts (M=1.39, SD=.96).
Frequency of using different websites
Participants were asked how often they have used different websites over the past two months. YouTube was the most visited website (M=2.68, SD=.93), followed by Facebook (M=2.59, SD=1.25). The least frequently used websites were TikTok (M=1.34, SD=.78) and Snapchat (M=1.13, SD=.54).
Discussion
This study aimed to better understand online registry facilitators, barriers, and preferred communication channels of Black BHR participants. The major findings were (1) that reported facilitators centered around personal knowledge and altruism; (2) that most reported no difficulties participating, but among those who did, the main barrier was time; and (3) email, YouTube, and Facebook are popular channels to reach and engage with Black adults.
Key factors that facilitated joining and continuing to participate BHR were related to personal benefits and altruism. The most endorsed reason for both joining and continuing was a desire to learn more about their own brain health. Similarly, when asked about additional features for BHR, participants were interested in gaining feedback about their participation (e.g., score on neuropsychological tests). This is consistent with previous research which identified that providing benefits (including test results) to the participant helps improve research participation (17, 31-33). Providing timely and continued feedback or information about participants’ brain health addresses the identified facilitators for joining and continuing to engage in research and may offset the cost and/or burden of participation and potentially make participants feel respected and understood. This finding highlights an important shortcoming of the BHR – not providing the participants with any results or feedback about their completed questionnaires and tests, which could provide them with information about their own brain health. While disclosure of neuropsychological test results, genetic results, and amyloid PET scans is currently a topic of great interest and debate in AD in-clinic studies (31, 34, 35), less research has focused on providing results or feedback in a remote setting without the presence of a clinician. When deciding to provide results in a remote setting, researchers must consider technical, logistical (e.g., features of the disclosure protocol), ethical, and safety issues (e.g., misinterpretation of results, psychological distress) and concerns about legal liability (31, 36, 37). In addition, many commonly used instruments are developed and tested in a non-Latinx White population, so they may not be valid in participants from diverse backgrounds. This threat to validity needs to be taken into account even when providing results in-person with a clinician and is heightened in an online setting without an involved clinician (38). Some studies are already providing results to participants, for example, the Alzheimer’s Prevention Trial Webstudy (APT Webstudy) is providing their registry participants with cognitive test scores and graphical representations of longitudinal performance (39). Another way of offering participants information about their brain health is through providing educational material which could be general or personalized.
Another key facilitator identified in this survey was altruism. In terms of altruism, the desire to help future generations and advancing science were often cited as reasons for participation. Altruism is also a commonly reported motivator for participating in research in general and the Black community in particular (17, 18, 33, 40-43). Follow-up studies need to determine whether inclusion and engagement materials focusing on providing feedback and altruism are successful in enrolling and engaging participants from the Black community in digital and in-clinic research over a period of time.
Regarding barriers to BHR participation, most survey respondents stated to have no difficulties participating. This result is not surprising considering that only a small and previously engaged number of Black BHR participants completed this survey. Yet it greatly limits our findings since we did not acquire the perceptions of Black adults who we failed to include (both in BHR in general and this survey). This is an important gap which future BHR research and research in general needs to continue to explore and address, through broader reach such as focus groups and nationally-representative surveys (44). Of those who experienced difficulties, the main perceived barriers were related to time. Participants reported both a lack of time and that BHR tasks were too time intensive, which is consistent with previous research findings (17, 45). Possible ways to address this issue in the BHR and other studies is by shortening assessments, being upfront about the time burden (e.g., provide information about percent completed), and giving participants the opportunity to break up the assessments into multiple sessions. The BHR website has recently undergone changes to address this issue by streamlining tasks. We have not yet determined if these changes will have a significant impact on the experience of BHR participants.
The most preferred way of receiving information from BHR was email, followed by text. Currently BHR only communicates via email with participants. To be able to include text communication and support in research studies, significant development work is needed. Considerations for text expansion include, for example, the management of participants whose phone numbers change and the appropriateness of text content for variable cell phone displays. In terms of website use, participants reported to most frequently use YouTube followed by Facebook. These results are consistent with a report from the Pew Research Center (46) in which both online platforms (Facebook and YouTube) rank highest among older adults and individuals from Black communities. However, evidence about the use of YouTube as a recruitment and inclusion tool is still in its infancy (47, 48). Previous BHR efforts to engage with potential participants, including commonly under-included ethnocultural groups, have primarily focused on Facebook, Google, and Bing (22, 49). As part of another research study, BHR is currently testing and comparing Facebook, Google, and YouTube ads tailored to Asian, Black, and Latinx adults, but the results about the effectiveness of this approach are still outstanding. Based on the current results, YouTube is an additional channel for future digital inclusion efforts among the Black community. This could include YouTube channels with videos about studies or laboratories, as well as digital advertisement placed within existing videos.
Limitations
This study is not without limitations. This study suffers from multiple selection biases including for those with internet access, ability to read and write in English, high literacy, those already enrolled in the BHR, and those responding to the survey invitation. Like many research samples, this study also underrepresents male participants and individuals with an education attainment less than a high school degree. Further, aside from education, BHR does not collect any other socioeconomic (e.g., income, quality of education) and sociocultural factors (e.g., discrimination) which could impact responses. This greatly impacts the interpretation and generalizability of our findings, and it remains unclear whether our results are applicable to a broader segment of Black community, especially those most underrepresented and underserved who are at greatest risk for AD/ADRD. Future efforts should focus on reaching adults from the Black community not currently enrolled in BHR or other research registries to understand the facilitators and barriers and help put into context the results from the present study. Furthermore, this analysis did not include information about the original recruitment (e.g., social media) source to BHR. Future research would benefit from including this information to help provide more context to the results regarding the participant’s frequency of using certain websites. Another limitation is the relatively low survey completion rate (5.4%). In BHR, enrollment rates for BHR referral studies are on average 10%, which is higher than the current study. However, the average referral study enrollment rate of 10% is across all participants and not specifically for Black BHR participants. In a previous analysis we found that BHR is less successful in enrolling non-White BHR participants in BHR referral studies compared to White BHR participants (26). In addition, only participants enrolled in the CEDAR study were presented with CEDAR survey. Of those who enrolled in CEDAR, 91.4% answered the survey. Future BHR survey studies could be sent to participants directly instead of requiring enrollment in an engagement study. The BHR is actively developing efforts to respond to these limitations.
Conclusion
In conclusion, our study provides insight into facilitators and barriers to online registry participation experienced by Black BHR participants, as well as their preferred modes of communications. These results will inform the development of culturally-informed efforts to increase the participation (enrollment and registry task completion) of Black BHR participants and can assist other researchers and developers in refining and improving their online registries or research studies. Overall, providing participants with feedback about their registry performance and information about their brain health, as well as reducing the time burden are among the recommended strategies to facilitate research engagement of Black participants. More work is needed to understand facilitators and barriers from a more diverse community of Black adults (e.g., gender, socioeconomic status) and Black adults who are not already enrolled in a study.
Funding: Funding for the project was received from the Genentech Health Equity Innovations 2020 Fund (G-89294 01/01/21–12/31/22, MPIs: M. Rivera Mindt, R. Nosheny). The sponsors had no role in the design and conduct of the study; in the collection, analysis, and interpretation of data; in the preparation of the manuscript; or in the review or approval of the manuscript.
Disclosure Statements: Danqi Zhu, Dr. Heining Cham, Jessica Bride, Emily McLean, Xinyue Deng Anna Aaronson, Catherine Conti, Carole Cypress, Philip Griffin, Regene Ross, Terrance Duncan, Aaron Ulbricht, Derek Flenniken, Diana Truran, Juliet Fockler, Monica Camacho, Dr. R. Scott Mackin, Dr. Carl V. Hill, and Dr. Robert Turner have nothing to disclose. Dr. Ashford reports grants from NIA during the conduct of the study (F32 AG072730-02). Dr. Byrd reports grants from Genetech during the conduct of the study. Dr. Weiner reports grants from National Institutes of Health (NIH), grants from Department of Defense (DOD), grants from Patient-Centered Outcomes Research Institute (PCORI), grants from California Department of Public Health (CDPH), grants from University of Michigan, grants from Siemens, grants from Biogen, grants from Hillblom Foundation, grants from Alzheimer’s Association, grants from The State of California, grants from Johnson & Johnson, grants from Kevin and Connie Shanahan, grants from GE, grants from VUmc, grants from Australian Catholic University (HBI-BHR), grants from The Stroke Foundation, grants from Veterans Administration, personal fees from Acumen Pharmaceutical, personal fees from Cerecin, personal fees from Dolby Family Ventures, personal fees from Eli Lilly, personal fees from Merck Sharp & Dohme Corp., personal fees from National Institute on Aging (NIA), personal fees from Nestle/Nestec, personal fees from PCORI/PPRN, personal fees from Roche, personal fees from University of Southern California (USC), personal fees from NervGen, personal fees from Baird Equity Capital, personal fees from BioClinica, personal fees from Cytox, personal fees from Duke University, personal fees from Eisai, personal fees from FUJIFILM-Toyama Chemical (Japan), personal fees from Garfield Weston, personal fees from Genentech, personal fees from Guidepoint Global, personal fees from Indiana University, personal fees from Japanese Organization for Medical Device Development, Inc. (JOMDD), personal fees from Medscape, personal fees from Peerview Internal Medicine, personal fees from Roche, personal fees from T3D Therapeutics, personal fees from WebMD, personal fees from Vida Ventures, personal fees from The Buck Institute for Research on Aging, personal fees from China Association for Alzheimer’s Disease (CAAD), personal fees from Japan Society for Dementia Research, personal fees from Korean Dementia Society, outside the submitted work; and I hold stocks or options with Alzheon Inc., Alzeca, and Anven. Dr. Rivera Mindt reports grants from NIH/NIA Pending # 1R01AG079285-01 , during the conduct of the study; grants from U19AG078109-01; R01AG066471; R56AG075744; R13AG071313-01; & R01AG065110 – 01A1; SC3GM141996; Genentech Health Equity Innovations 2020 Fund G-89294, outside the submitted work. Dr. Nosheny reports grants from NIH, grants from Genentech, Inc., grants from California Department of Public Health during the conduct of the study.
Acknowledgements: We would like to acknowledge all survey respondents and the hard work, insightful comments from all CEDAR Community-Science Partnership Board members including: Bernadette Waddell, Anna Bell Middleton, Philip Griffin, and Veronicia Lee. We would also like to acknowledge and thank the funder (Genentech) and all BHR team members.
Ethical standards: The UCSF Institutional Review Board approved the survey.
Open Access: This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
References
1. Association, A.s., 2020 Alzheimer’s Disease Facts and Figures. Alzheimers Dement, 2020. 16(3): p. 391. https://doi.org/10.1002/alz.12068
2. Mehta, P., et al., CDC Grand Rounds: National Amyotrophic Lateral Sclerosis (ALS) Registry Impact, Challenges, and Future Directions. MMWR Morb Mortal Wkly Rep, 2017. 66(50): p. 1379-1382. https://doi.org/10.15585/mmwr.mm6650a3
3. Tang, M.-X., et al., The APOE-ε 4 allele and the risk of Alzheimer disease among African Americans, whites, and Hispanics. Jama, 1998. 279(10): p. 751-755. https://doi.org/10.1001/jama.279.10.751
4. Stites, S.D., et al., Establishing a framework for gathering structural and social determinants of health in Alzheimer’s disease research centers. The Gerontologist, 2022. 62(5): p. 694-703. https://doi.org/10.1093/geront/gnab182
5. Barnes, L.L., et al., Change in cognitive function in Alzheimer’s disease in African-American and white persons. Neuroepidemiology, 2006. 26(1): p. 16-22. https://doi.org/10.1159/000089231
6. Green, R.C., et al., Risk of dementia among white and African American relatives of patients with Alzheimer disease. Jama, 2002. 287(3): p. 329-336. https://doi.org/10.1001/jama.287.3.329
7. Hendrie, H.C., et al., Incidence of dementia and Alzheimer disease in 2 communities: Yoruba residing in Ibadan, Nigeria, and African Americans residing in Indianapolis, Indiana. Jama, 2001. 285(6): p. 739-747. https://doi.org/10.1001/jama.285.6.739
8. Shadlen, M.F., et al., Education, cognitive test scores, and black-white differences in dementia risk. Journal of the American Geriatrics Society, 2006. 54(6): p. 898-905. https://doi.org/10.1111/j.1532-5415.2006.00747.x
9. Turner, A.D., et al., Perceived stress and cognitive decline in different cognitive domains in a cohort of older African Americans. The American Journal of Geriatric Psychiatry, 2017. 25(1): p. 25-34. https://doi.org/10.1016/j.jagp.2016.10.003
10. Wilson, R.S., et al., Factors related to racial differences in late-life level of cognitive function. Neuropsychology, 2016. 30(5): p. 517. https://doi.org/10.1037/neu0000290
11. Barnes, L.L., Alzheimer disease in African American individuals: increased incidence or not enough data? Nature Reviews Neurology, 2022. 18(1): p. 56-62. https://doi.org/10.1038/s41582-021-00589-3
12. Fargo, K.N., et al., The crisis in recruitment for clinical trials in Alzheimer’s and dementia: An action plan for solutions. Alzheimer’s & Dementia, 2016. 12(11): p. 1113-1115. https://doi.org/10.1016/j.jalz.2016.10.001
13. Shin, J. and P.M. Doraiswamy, Underrepresentation of African-Americans in Alzheimer’s trials: a call for affirmative action. Frontiers in aging neuroscience, 2016. 8: p. 123. https://doi.org/10.3389/fnagi.2016.00123
14. Birkenbihl, C., et al., Evaluating the Alzheimer’s disease data landscape. Alzheimer’s & Dementia: Translational Research & Clinical Interventions, 2020. 6(1): p. e12102. https://doi.org/10.1002/trc2.12102
15. Franzen, S., et al., Diversity in Alzheimer’s disease drug trials: The importance of eligibility criteria. Alzheimer’s & Dementia, 2022. 18(4): p. 810-823. https://doi.org/10.1002/alz.12433
16. Kennedy, R.E., et al., Challenging assumptions about African American participation in Alzheimer disease trials. The American Journal of Geriatric Psychiatry, 2017. 25(10): p. 1150-1159. https://doi.org/10.1016/j.jagp.2017.04.013
17. George, S., N. Duran, and K. Norris, A systematic review of barriers and facilitators to minority research participation among African Americans, Latinos, Asian Americans, and Pacific Islanders. American journal of public health, 2014. 104(2): p. e16-e31. https://doi.org/10.2105/AJPH.2013.301706
18. Hughes, T.B., et al., African Americans and clinical research: evidence concerning barriers and facilitators to participation and recruitment recommendations. The Gerontologist, 2017. 57(2): p. 348-358. https://doi.org/10.1093/geront/gnv118
19. Tanner, A., et al., Barriers to medical research participation as perceived by clinical trial investigators: communicating with rural and African American communities. Journal of health communication, 2015. 20(1): p. 88-96. https://doi.org/10.1080/10810730.2014.908985
20. Airhihenbuwa, C.O. and C.L. Ford, Critical Race Theory–We are all others. Ethnicity & disease, 2018. 28(Suppl 1): p. 219. https://doi.org/10.18865/ed.28.S1.219
21. Lincoln, K.D., et al., Fundamental causes of barriers to participation in Alzheimer’s clinical research among African Americans. Ethnicity & health, 2021. 26(4): p. 585-599. https://doi.org/10.1080/13557858.2018.1539222
22. Weiner, M.W., et al., The Brain Health Registry: An internet-based platform for recruitment, assessment, and longitudinal monitoring of participants for neuroscience studies. Alzheimer’s & Dementia, 2018. 14(8): p. 1063-1076. https://doi.org/10.1016/j.jalz.2018.02.021
23. Grill, J.D., et al., Constructing a local potential participant registry to improve Alzheimer’s disease clinical research recruitment. Journal of Alzheimer’s Disease, 2018. 63(3): p. 1055-1063. https://doi.org/10.3233/JAD-180069
24. Langbaum, J.B., et al., The Alzheimer’s Prevention Registry’s Genematch program: update on progress and lessons learned in helping to accelerate enrollment into Alzheimer’s prevention studies. Alzheimers and Dementia, 2018. 14(7): p. P1073. https://doi.org/10.1016/j.jalz.2018.06.1379
25. Ashford, M.T., et al., Underrepresented Elders in The Brain Health Registry: US Representativeness and Registry Behavior, in Clinical Trials and Aging: 12th Conference Clinical Trials on Alzheimer’s Disease. 2019, The Journal of Prevention of Alzheimer’s Disease (JPAD). San Diego. p. 45–154.
26. Ashford, M.T., et al., Effects of sex, race, ethnicity, and education on online aging research participation. Alzheimer’s & Dementia: Translational Research & Clinical Interventions, 2020. 6(1): p. e12028. https://doi.org/10.1002/trc2.12028
27. Nosheny, R.L., et al., Online study partner-reported cognitive decline in the Brain Health Registry. Alzheimer’s & Dementia: Translational Research & Clinical Interventions, 2018. 4: p. 565-574. https://doi.org/10.1016/j.trci.2018.09.008
28. Mackin, R.S., et al., Unsupervised online neuropsychological test performance for individuals with mild cognitive impairment and dementia: Results from the Brain Health Registry. Alzheimer’s & Dementia: Diagnosis, Assessment & Disease Monitoring, 2018. 10: p. 573-582. https://doi.org/10.1016/j.dadm.2018.05.005
29. Team, R.C., R: language and environment for statistical computing. 2017, R Foundation for Statistical Computing: Vienna, Austria.
30. Braun, V. and V. Clarke, Using thematic analysis in psychology. Qualitative research in psychology, 2006. 3(2): p. 77-101. https://doi.org/10.1191/1478088706qp063oa
31. Roberts, J.S., et al., Disclosure of individual research results at federally funded Alzheimer’s Disease Research Centers. Alzheimer’s & Dementia: Translational Research & Clinical Interventions, 2021. 7(1): p. e12213. https://doi.org/10.1002/trc2.12213
32. Avent, C., et al., Establishing the motivations of patients with dementia and cognitive impairment and their carers in joining a dementia research register (DemReg). International psychogeriatrics, 2013. 25(6): p. 963-971. https://doi.org/10.1017/S1041610213000252
33. Sano, M., et al., Participant satisfaction with dementia prevention research: Results from Home-Based Assessment trial. Alzheimer’s & Dementia, 2018. 14(11): p. 1397-1405. https://doi.org/10.1016/j.jalz.2018.05.016
34. Erickson, C.M., et al., Disclosure of preclinical Alzheimer’s disease biomarker results in research and clinical settings: why, how, and what we still need to know. Alzheimer’s & Dementia: Diagnosis, Assessment & Disease Monitoring, 2021. 13(1): p. e12150. https://doi.org/10.1002/dad2.12150
35. Rosen, A.C., et al., The Advisory Group on Risk Evidence Education for Dementia: Multidisciplinary and Open to All. Journal of Alzheimer’s Disease, 2022(Preprint): p. 1-9. https://doi.org/10.3233/JAD-220458
36. Grill, J.D. and J. Karlawish, Disclosing Alzheimer Disease Biomarker Results to Research Participants. JAMA neurology, 2022. https://doi.org/10.1001/jamaneurol.2022.1307
37. Harkins, K., et al., Development of a process to disclose amyloid imaging results to cognitively normal older adult research participants. Alzheimer’s research & therapy, 2015. 7(1): p. 1-9. https://doi.org/10.1186/s13195-015-0112-7
38. Rivera Mindt, M., et al., Increasing culturally competent neuropsychological services for ethnic minority populations: A call to action. The Clinical Neuropsychologist, 2010. 24(3): p. 429-453. https://doi.org/10.1080/13854040903058960
39. Aisen, P., et al., The trial-ready cohort for preclinical/prodromal Alzheimer’s disease (TRC-PAD) project: an overview. The journal of prevention of Alzheimer’s disease, 2020. 7(4): p. 208-212. https://doi.org/10.14283/jpad.2020.45
40. Williams, M.M., et al., Barriers and facilitators of African American participation in Alzheimer’s disease biomarker research. Alzheimer disease and associated disorders, 2010. 24(Suppl): p. S24. https://doi.org/10.1097/WAD.0b013e3181f14a14
41. Drake, B.F., et al., Barriers and strategies to participation in tissue research among African-American men. Journal of Cancer Education, 2017. 32(1): p. 51-58. https://doi.org/10.1007/s13187-015-0905-1
42. Gilmore-Bykovskyi, A.L., et al., Recruitment and retention of underrepresented populations in Alzheimer’s disease research: a systematic review. Alzheimer’s & Dementia: Translational Research & Clinical Interventions, 2019. 5: p. 751-770. https://doi.org/10.1016/j.trci.2019.09.018
43. Lee, J.Y., et al., “If it helps someone, then I want to do it”: Perspectives of persons living with dementia on research registry participation. Dementia, 2020. 19(8): p. 2525-2541. https://doi.org/10.1177/1471301219827709
44. Bleakley, A., et al., An Elicitation Study to Understand Black, Hispanic, and Male Older Adults’ Willingness to Participate in Alzheimer’s Disease-Focused Research Registries. Journal of Alzheimer’s Disease, 2022(Preprint): p. 1-11. https://doi.org/10.3233/JAD-220196
45. Renfrew, M.E., et al., Participant perceptions of facilitators and barriers to adherence in a digital mental health intervention for a nonclinical cohort: content analysis. Journal of Medical Internet Research, 2021. 23(4): p. e25358. https://doi.org/10.2196/25358
46. Auxier, B. and M. Anderson, Social Media Use in 2021. 2021.
47. Harris, P.A., et al., ResearchMatch: a national registry to recruit volunteers for clinical research. Academic medicine: journal of the Association of American Medical Colleges, 2012. 87(1): p. 66. https://doi.org/10.1097/ACM.0b013e31823ab7d2
48. Culshaw, S., YouTube as a Recruitment Tool? A Reflection on Using Video to Recruit Research Participants: Profiling Emerging Research Innovations. Video Journal of Education and Pedagogy, 2020. 5(1): p. 1-19. https://doi.org/10.1163/23644583-00501004
49. Ashford, M.T., et al., Digital culturally tailored marketing for enrolling Latino participants in a web-based registry: Baseline metrics from the Brain Health Registry. Alzheimer’s & Dementia, 2022. https://doi.org/10.1002/alz.12805
© The Authors 2023