S.B. Hendrix, S.P. Dickson
Pentara Corporation
Corresponding Author: Suzanne B. Hendrix Pentara Corporation, Salt Lake City, UT, USA, shendrix@pentaracorp.com
J Prev Alz Dis 2023;1(10):7-8
Published online December 22, 2022, http://dx.doi.org/10.14283/jpad.2022.104
The authors have done a nice job applying an anchor-based approach to determining a clinically meaningful change on scales commonly used in mild cognitive impairment due to Alzheimer’s disease (1). They have clearly articulated the limitations of these results and have appropriately emphasized that these thresholds are not minimally clinical important effects and should not be applied to populationlevel treatment differences. Despite this caution, there will be readers who misapply these thresholds due to misunderstandings of these types of calculations, which are common. They have also noted that these methodologies are appropriate when the ICC is above the commonly used threshold of 0.7, and have acknowledged that the CDR-sb and ADAS-cog 11 are both slightly below this cutoff suggesting that these outcomes are in the range where this methodology is less robust than the usual circumstances. Despite these limitations, these thresholds have useful applications for responder analysis cutoffs in clinical trials.
Anchor-based methodology itself also has limitations. Determining per-person clinically meaningful change in this way is based on the assumption that the categories of the anchoring scale are clinically meaningfully different from each other, which they are, but also end up being interpreted as if smaller changes are not clinically meaningful, which is usually not established. These changes may also be larger than values considered meaningful to patients and/or caregivers (as noted by the authors). In other words, the anchor scales may not have minimally clinically important point categories, and this method will never be able to identify clinically meaningful changes that are smaller than the points on the anchoring scales.
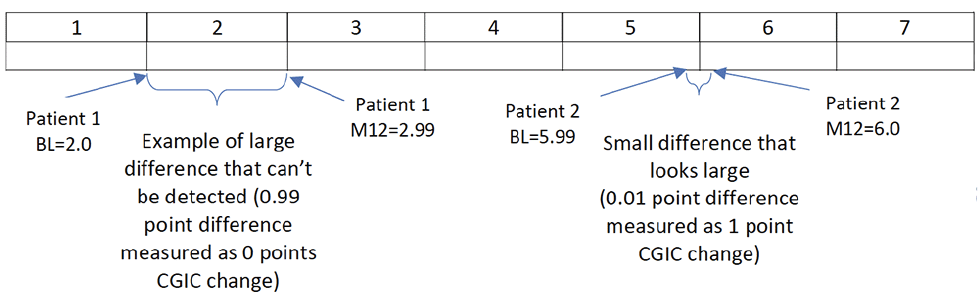
The authors also point out the fact that the broad categories of the anchoring scales, although intended to detect large, meaningful change, also have the unintended consequence that individuals near the boundary of 2 scores may change only a small amount but get credited for a large and meaningful effect. Conversely, individuals who change by a large amount from the low end of a single score to the high end of that same score on the anchoring scale, may show no change at all despite experiencing nearly a point of change on the scale (see figure 1).
Interestingly, the two anchoring scales that were used in this study utilize different methodologies for establishing clinically meaningful categories of response. This is noted by the authors but is worth reemphasizing. ADCS-CGIC-MCI is assessed relative to baseline, and GDS is an absolute scale. This distinction is critical since calculating a change from baseline introduces error at both baseline and follow-up for the GDS. In contrast, assessing a change from baseline clinically, as is done with the ADCSCGIC-MCI may have implicit error due to comparing to a recalled baseline evaluation, but avoid the introduction of additional measurement error. It is interesting that similar results were found with both anchors.
The anchor-based methodology for determining clinical meaningfulness has challenges particularly as it applies to a degenerative disease with high day-to-day variability (low ICC). This makes it particularly inappropriate to apply these cutoffs to an individual in the clinic to determine per-person meaningful changes since expected day-to-day variability may be overinterpreted.
A survival analysis of time to (first) event could be used with these cutoffs for defining an event, but in this case, expected variability will be interpreted as meaningful change since a patient who responds is not observed for “loss” of that event after it occurs the first time. The best use of these thresholds is for definitions of responders in a clinical trial, since this is a per-person application that takes advantage of population distributions to compensate for the variability. Some people will be unusually high on one day and others unusually low and with a population, these will balance each other out. Using these thresholds for responder analyses will roughly correspond to using an ADCS-CGIC-MCI point or a GDS point as a response criterion.
Conflict of interest: SBH is owner and employee of Pentara Corporation and SPD is an employee of Pentara Corporation. Pentara performs statistical analyses for dozens of companies as a paid consultant in the Alzheimer’s disease space.
Reference
1. Lansdall CJ, McDougall F, Butler LM, Delmar P, Pross N, Qin S, McLeod L, Zhou X, Kerchner GA, Doody RS. Establishing clinically meaningful change on outcome assessments frequently used in trials of mild cognitive impairment due to Alzheimer’s disease. J Prev Alz Dis 2023;1(10):9-18; Doi: http://dx.doi.org/10.14283/jpad.2022.102
© Serdi 2022