O.A.R. Abozaid1, M.W. Sallam1, E.S.A. Ahmed2
1. Biochemistry Department, Faculty of Veterinary Medicine, Benha University, Egypt; 2. Radiation Biology Research, National Center for Radiation Research and Technology, Atomic Energy Authority, Cairo, Egypt..
Corresponding Author: Esraa S.A. Ahmed, Egyptian Atomic Energy Authority, Nasr City, Cairo 11787, Egypt, E-mail: esraa.tamim@yahoo.com, https://orcid.org/0000-0001-5358-4394
J Prev Alz Dis 2022;3(9):458-468
Published online February 18, 2022, http://dx.doi.org/10.14283/jpad.2022.26
Abstract
Prolonged exposure to environmental aluminum-containing substances is associated with the development of Alzheimer’s disease (AD). AD is a brain disorder associated with a gradual weakening in neurocognitive functions. Mesenchymal stem cells (MSCs) transplant as a promising and safe approach is used to treat AD through countless mechanisms. Therefore, this study aims to elucidate how MSCs improve biochemical and histopathological approaches associated with the AD model in rats. MSCs treatment restores the redox status impairment through a notable decline in the malondialdehyde (MDA) levels along with antioxidant enrichment. The anti-inflammatory effect of MSCs through conversion of microglial cells from M1 to M2 and inhibition of pro-inflammatory mediator’s release work in with de-activated GSK-3β. Additionally, the alleviation of autophagy and lysosomal clearance of Aβ and tau aggregates was accompanied by a down-regulation of the mTOR. Moreover, MSCs upregulate the expression of SIRT1 together with a limited expression of miR-134 thereby, improve neurite outgrowth and synaptic loss. Overall, the obtained data confirm the novelty of MSCs in the treatment of AD not only by their antioxidant, anti-inflammatory effect but also by restoring the neural integrity, neurogenesis, improving the neurocognitive function, and modulation of the signal pathways linked to the Aβ hypothesis.
Key words: Aluminium, Alzheimer’s disease, mesenchymal stem cells, amyloid β, SIRT1, miR-134.
Abbreviations: Al: Aluminium; AD: Alzheimer’s disease; Aβ: amyloid β; MSCs: mesenchymal stem cells; SIRT: Sirtuin; AlCl3: Aluminum chloride; MDA: malondialdehyde; CAT: catalase; H2O2: hydrogen peroxide; GSH: glutathione; IL-1β: Interleukin- 1β; ANOVA: One-way analysis of variance; LSD: Least Significant Difference; SE: standard error; STAT3: signal transducer and activator of transcription; PI3K: phosphatidylinositol 3 kinase; GSK3β: glycogen synthase kinase 3 beta; mTOR: mammalian target of rapamycin; MiR-134 microRNA- 134.
Introduction
Aluminium (Al) is a highly widespread environmental and industrial toxicant (1). The extensive exposure and use of the Al in daily life through inhalation, ingestion of food, drinking water, drugs, and during hemodialysis and vaccination increase its intoxications in the human body. Al causes toxicity in various organs including the liver, and most importantly in the nervous system such as Alzheimer’s disease (AD) (2). Excessive accumulation of AlCl3 in the liver tissue caused cellular degeneration, changes in the permeability of hepatic cell membranes, and finally impaired hepatic function (3). Due to the ability of aluminum to cross the blood-brain barrier (BBB), it is one of the potent neurotoxin agents that are involved in the pathogenesis of AD usually mediating direct and indirect mechanisms of action (4). Previous studies indicated that aluminum accumulates in various areas of the brain leading to the accumulation of the most essential biomarkers for AD pathology P-tau and amyloid β. This initiate various cascade including neurotoxicity and neuroinflammation. Moreover, aluminum chloride leads to oxidative stress and deterioration of cellular lipid proteins and DNA and impaired cholinergic transmission, apoptotic neuronal death (5). Furthermore, Kumar et al. (6) confirm that aluminum induces cognitive deficits via hippocampus disorientation, memory, and synaptic plasticity impairments, and ultimately dementia which is similar to the pathogenesis of AD. Therefore, AlCl3 is used as an established AD animal model to investigate the efficacy of therapeutic agents for AD.
AD is a neurodegenerative disorder identified with a progressive impairment of mental function and the loss of synapsis and neurons involved in learning, and memory. In AD, the neurotoxicity and pathogenesis resulted from an extreme accumulation of misfolded proteins (hyper-phosphorylated tau protein and amyloid β Aβ-42) (7), oxidative stress injury, mitochondrial dysfunction, neuroinflammation, autophagy, and impaired signaling pathways (8).
Mesenchymal stem cells (MSCs) are a group of self-renewing multipotent stem cells, easily collected from many tissues such as bone marrow (9, 10). MSCs can effectively cross the blood-brain barrier (BBB), avoid immune rejection and improve patients’ quality of life (11). The ability of MSCs to migrate and home into the injured tissues and their multidirectional differentiation into neural cells not only regenerate impaired neuronal tissue but also inhibit the disease progression. Moreover, MSCs through their paracrine effects secrete neurotrophic factors, like BDNF and GDNF, which stimulate endogenous neurogenesis, modulate neuroplasticity and activate microglia or differentiation into microglia cells (12). MSCs differentiate not only to neural cells but also into multiple cell lineages, including hepatocytes, that can promote liver regeneration, reduce liver injury and improve liver function (13, 14).
The Sirtuin (SIRT) family are actively involved in preventing cell senescence and prolonging the lifespan of an organism by modulating various cell biological processes, including apoptosis, transcription, neurogenesis, inflammation, and aging (15). Reduced activity of SIRT is associated with abnormal brain development. As a member of the SIRT family, SIRT1 is highly expressed in brain neurons and is widely attributed to a general role against neurodegeneration (16). Synaptic plasticity and memory are regulated through SIRT1/ microRNA mechanism (17). MicroRNAs, a class of non-coding RNAs, modulate the post-transcriptional gene expression through translation blunt or mRNAs degradation (18). MiRNAs play important roles in brain maturation, neuron development and differentiation, and synaptic plasticity (19). Liu et al. (20) reported that microRNAs have a role in the regulation of the Aβ hypothesis (Aβ production and clearance) and the tau hypothesis (tau hyperphosphorylation and formation of neurofibrillary tangles) that is involved in the progression of AD. Consequently, the deregulation of miRNAs causes neurodegenerative diseases and affects processes of synaptogenesis, inflammation, and neurodegeneration (21). Consequently, we hypothesized to evaluate the possible therapeutic role of MSCs in the alleviation of neurotoxicity through a variety of mechanisms in a rat model of AD.
Materials and Methods
Materials
Chemicals
Aluminum chloride (AlCl3) was purchased from Elgomhoria company (Cairo, Egypt). All other chemicals used in the study were of analytical-reagent grade.
Methods
Isolation of BM- MSCs
From male Wistar rats (6-week-old) tibiae and femurs, the bone marrow was flushed using Dulbecco’s modified Eagle’s medium (DMEM, GIBCO/BRL) enriched with 10% fetal bovine serum (GIBCO/BRL). Using density gradient [Ficoll/Paque (Pharmacia)], the nucleated cells were isolated and were re-suspended in a complete culture medium containing 1% penicillin-streptomycin (GIBCO/BRL). After that, at 37° C and 5% humidified CO2 cells were incubated 50 cm2 culture flasks for 14 days. On day 14, the adherent cells and colonies were washed with phosphate buffer saline (PBS) twice and were harvested with trypsin (0.25% trypsin in 1 mM EDTA (GIBCO/BRL) and counted (22).
Identification of BM- MSCs
MSCs cells were characterized by their shape, adherence, and detection of their CDs using flow cytometry. The harvested cells were washed and re-suspended in PBS in aliquots of 106 cells. After that the cells were incubated with monoclonal antibodies, conjugated with fluorescence isothiocyanate (FITC), phycoerythrin (PE) in 250 μl phosphate-buffered saline for 30 min in dark at room temperature. The following cell surface antigens were observed CD90-FITC, CD34-FITC, CD105-PE (23). Mouse isotype-matched IgG is used as a negative control (BD Pharmingen). Using CellQuest software, 100,000 labeled cells were acquired and analyzed.
Experimental animals
Thirty male Wistar rats (6–7 weeks) were brought from the Animal Research Institute (Cairo, Egypt). In the experimental period, rats were allowed ad libitum access to food and water and housed under the same laboratory conditions with a light/dark cycle of 12 h, humidity of 50 ±15%, and temperature of 22 ±2 ºC. All experimental procedures were performed in compliance with the standards and guidelines of the National Research Centre Ethics Committee, issued by the U.S. National Institutes of Health, “Guide for the treatment and use of laboratory animals” for the utility and protection of experimental animals (NIH publication No. 85–23, 1996).
AlCl3 was dissolved in distilled water and was administered orally at a dose of 0.5 mL/100 g bodyweight.
Experimental design
After acclimatization for five days, the rats (100–150 g) were divided into three groups (n = 10) randomly as follows:
1. Group 1: normal control group (Negative Control).
2. Group 2: (AD): the AD group in which rats were orally administered with AlCl3 at a dose of 100 mg/kg/day for 60 days (24).
3. Group 3: (AD+ MSCs): the rats were orally administered with AlCl3 at a dose of 100mg/kg/day for 60 days and were transplanted with a single intravenous injection of BM-MSCs in the tail vein (106 cells/rat), then rats were left for one month.
After that, the animals were anesthetized via ip injection of 0.1 mL of urethane (1g/kg) and were sacrificed via cervical dislocation. An incision was made on the dorsal side of the skull and brains were collected, cleaned, and washed with saline (0.9% of sodium chloride) stored at −80°C for different estimates of biochemical parameters. Some brain tissues were rinsed in 10 % neutralized formalin for histopathological examination.
Biochemical estimations
Lipid peroxidation was measured as malondialdehyde (MDA) levels according to the method of Yoshioka et al. (25) and is reported as μmol/g of the brain. Brain catalase (CAT) activity was measured as consumption of H2O2 according to the method of Sinha (26) and is expressed as μmol/min/g of the brain. According to Beutler et al. (27) method, reduced glutathione (GSH) content was measured and is reported as μmol/g of the brain.
Amyloid β-protein (Aβ) and phosphorylated tau protein (p-tau) concentrations were determined by Rat amyloid beta-peptide 1-42 and Rat Phospho Tau Protein (PTAU) ELISA Kit according to the manufacture’s instruction (My Biosource Inc., San Diego California, USA). Interleukin- 1β (IL-1β) levels were determined by ELISA kit following the protocols provided by the manufacturers (RayBiotech Life, USA). Moreover, the levels of acetylcholine were determined by Quick Detect Acetylcholine (ACh) (Rat) ELISA Kit (BioVision, Inc. San Francisco, Milpitas, USA).
Estimation of liver function tests
Serum activities of aspartate transaminase (AST), alanine transaminase (ALT) as a liver function were determined according to the method of Reitman and Frankel (28).
Western immunoblotting
Brain tissue proteins were extracted using TRIzol reagent, and protein concentrations were quantified with the Bradford method (29). 20µg of protein per lane were separated with 10% SDS PAGE and blotted onto polyvinylidene difluoride membranes. After blocking by incubation for 2h with Tris-buffered saline (10 mM Tris-Cl, pH 7.5, 100 mM NaCl) containing 5% nonfat dried milk and 0.1% Tween 20, the membrane was probed with the primary antibodies towards PI3K, mTOR, STAT3, GSK3β and SIRT1 with β-actin as control. After washing, membranes were blotted with the secondary monoclonal antibodies conjugated to horseradish peroxidase at room temperature for 2h, and then membranes were washed four times with the same washing buffer. Using the Amersham detection kit according to the manufacturer’s protocols, the membranes were visualized by chemiluminescence after exposure to X-ray film. Primary and secondary antibodies were purchased from Cell Signaling Technologies, USA. By densitometric analysis of the autoradiograms using a scanning laser densitometer (Biomed Instrument Inc., USA) the proteins quantities were determined and were expressed as arbitrary units after normalization for β- actin.
Molecular Investigation
Determination of mRNA gene expression of SIRT1 and MiR-134 by Quantitative Real-Time PCR (qRT-PCR) in brain tissues
Isolation of RNA and Reverse Transcription: The change in mRNA expression of SIRT1 and MiR-134 was examined. Total RNA was isolated from brain tissues (30mg) via TRIzol reagent (Life Technologies, USA). Then the integrity of RNA was checked with1% agarose gel electrophoresis stained with bromide of ethidium. The synthesis of the first strand complementary DNA (cDNA) was done with reverse transcriptase (Invitrogen) using 1μg of total RNA as the template according to the manufacturer’s protocol.
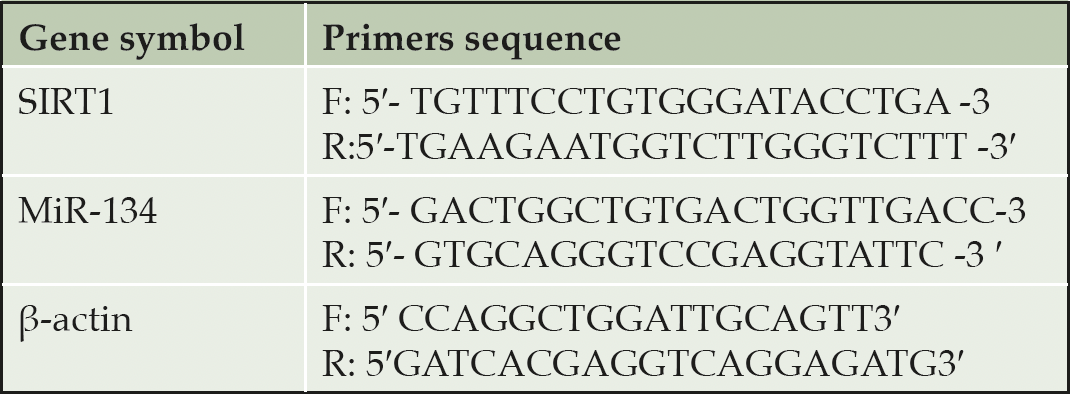
Quantitative Real-time Polymerase Chain Reaction (qPCR) RT-PCRs were performed using the Sequence Detection Program (PE Biosystems, CA) in a thermal cycler stage one plus (Applied Biosystems, USA). Table 1 lists the primer pairs used in these experiments. A 25μl total volume reaction mixture consisting of 2X SYBR Green PCR Master Mix (Applied Biosystems), 900 nM of each prim, and 2μL of cDNA. The conditions for PCR thermal cycling were adjusted as follow, the initial step at 95 ° C for 5 min; 40 cycles at 95 ° C for 20s; 60 ° C for 30s; and 72 ° C for 20s. Finally, the results were normalized using the β-actin gene. The relative expression of target mRNA was calculated using the method of comparative Ct (30).
Histopathological analysis
Brain tissues were fixed in 10 % formaldehyde solution and were inserted in paraffin using standard methods. The brain tissue sections (3-μm), were stained with hematoxylin-eosin (H&E) stain. Under a light microscope, the stained sections were examined for histopathological alterations at 400 X magnification.
Statistical analyses
The obtained data were represented as the mean ± SE. One-way analysis of variance (ANOVA) with Least Significant Difference (LSD) was used to check differences in means of variables between groups. A probability of P<0.05 was considered significant. All data were analyzed by Statistical Package for Social Science (SPSS) version 20 for Windows (SPSS® Chicago, IL, USA) software program.
Results
Isolation and flow cytometric analysis of BMSCs
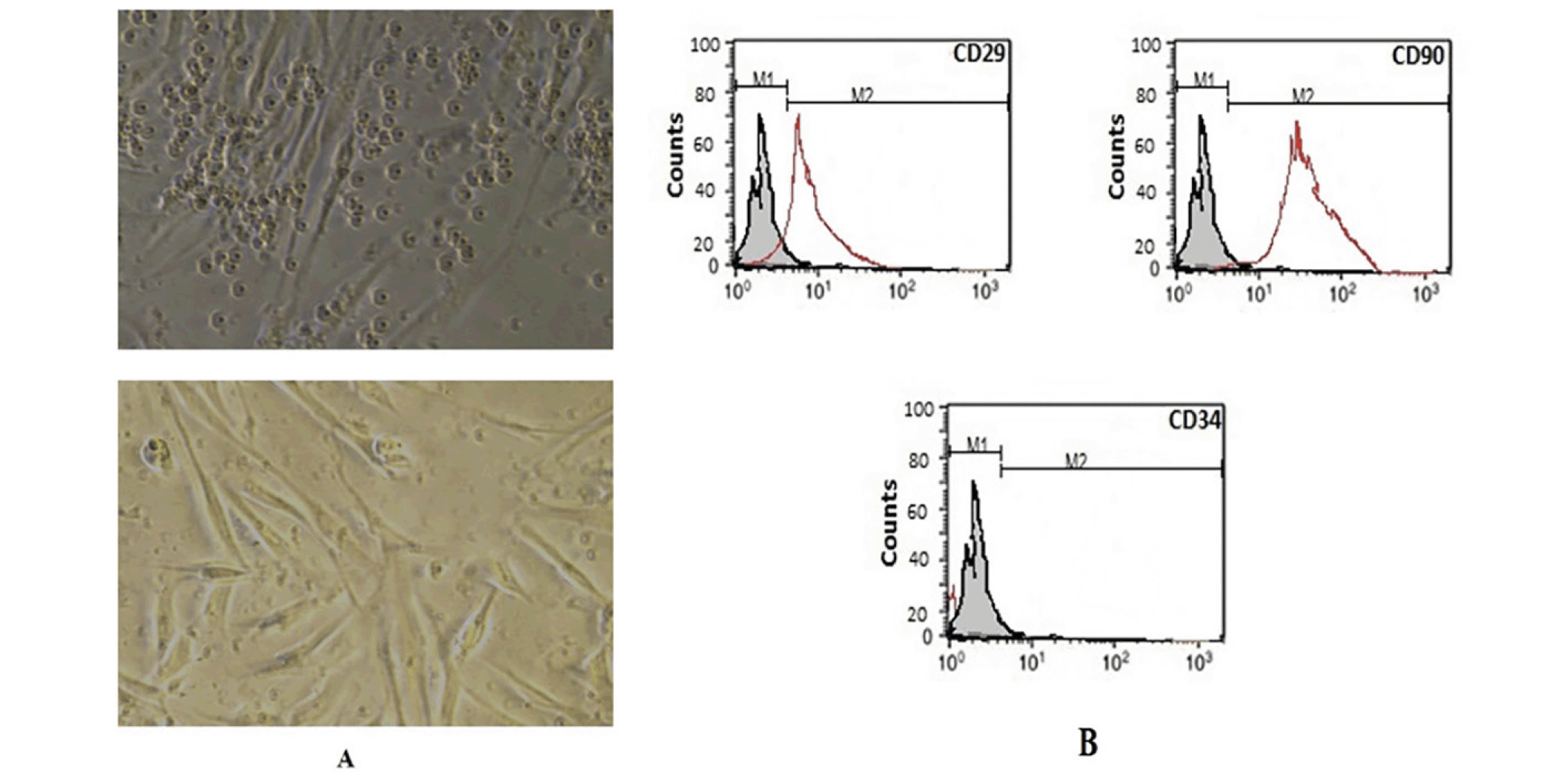
The Isolated and cultured undifferentiated BM-MSCs reached 70–80% confluence on day 14. BM-MSCs were identified by their adhesiveness and fusiform spindle shape (figure1, A). The flow cytometry charts showed that the BM-MSCs negatively expressed CD34 (≤ 4%), which is a cell surface marker associated with hematopoietic cells. Furthermore, BM-MSCs cells strongly expressed CD29 and CD90 (≥ 90 %), which are important cell surface markers of MSCs as illustrated in Figure (1, B).
Undifferentiated MSCs (A) revealed a flat fibroblast-like morphology under inverted microscopy. According to the results of flow cytometric analysis of BM-MSCs (B), they had positive values for CD90 and CD29 and negative values for CD34.
Effect of MSCs treatment on brain oxidative stress status in an AD rat model
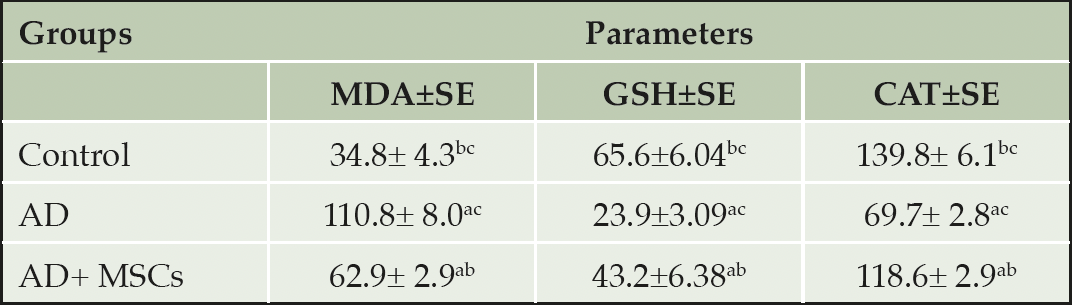
Mitochondrial dysfunction associated with oxidative damage potentiates aluminum-induced neurotoxicity. AlCl3 supplementation to rats resulted in severe toxicity in the brain tissues accompanied with impairment of the oxidative stress/ antioxidant status. Statistical data in table 2 depicted a marked increase in the level of brain oxidative stress marker (MDA) coupled with a significant decline in the content and activities of antioxidant (GSH and CAT) in the AlCl3 treated group when compared with the control. However, treatment with MSCs significantly reduced MDA level as well as enhanced GSH content and CAT activity respectively in the brain when compared to AlCl3-treated animals.
Values were expressed as Means ± SE, (n=6). a, b, and c denote significant change at p<0.05 versus control, AD, and MSCs groups, respectively.
Effects of MSCs on Aβ-42 tau proteins concentrations in the brain of the AD rat model
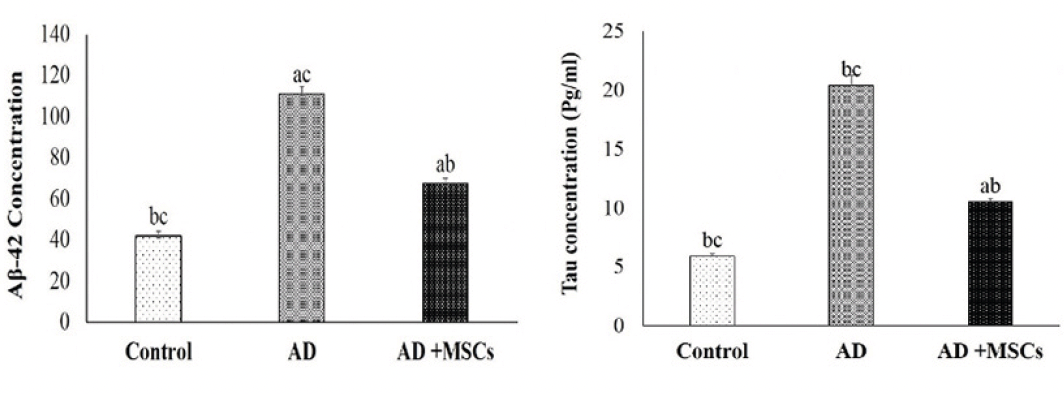
The abnormal alteration of aggregated proteins (amyloid β-protein (Aβ) and hyperphosphorylation of tau protein (p-tau)) in the brain of the AlCl3 treated rats is represented in figure (2). Prolonged administration of AlCl3 significantly upregulated the levels of both Aβ-42 and p-tau in the brain tissues of the AD model as compared to the control group. On the contrary, treatment with MSCs resulted in a marked decline of these aggregated proteins compared to the untreated AD group.
Data are presented as the means ± SE (n= 6), a, b and c denote significant change at p<0.05 versus control, AD and MSCs groups, respectively.
Effects of MSCs on the AlCl3 induced neuroinflammation in rats
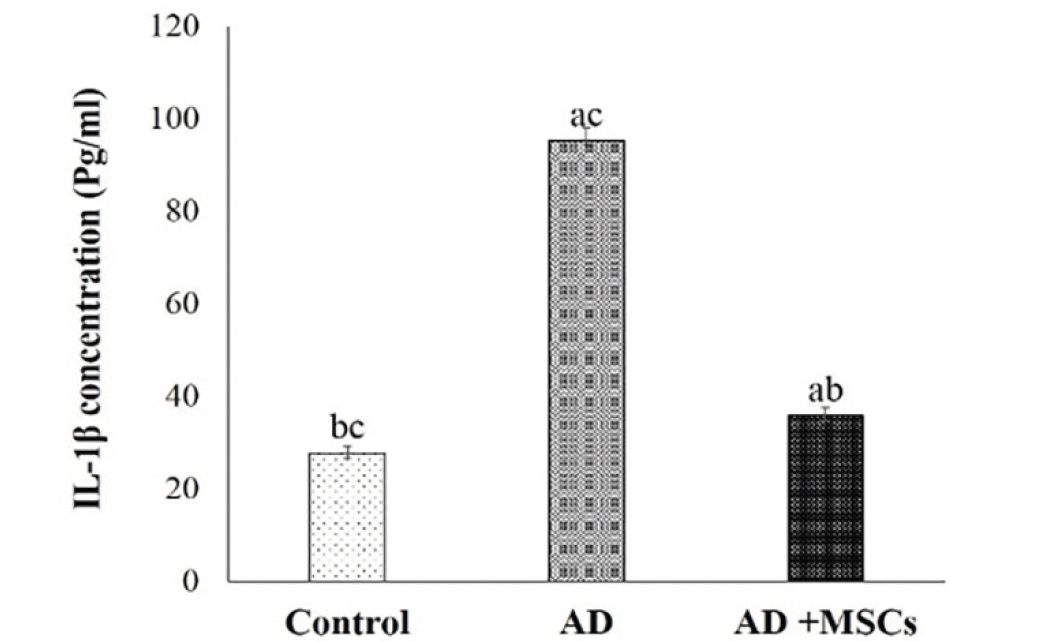
Microglia plays a crucial role in the development of the brain. However, exposure to Al activates microglial cells causing neurotoxicity and neuroinflammation through the production of pro-inflammatory cytokines (IL-1β). As shown in figure (3), the levels of IL-1β in the brain were markedly increased in the AlCl3-treated rats. Meanwhile, treatment with MSCs significantly reversed the effect of AlCl3.
Data are presented as the means ± SE (n= 6). a, b and c denote significant change at p<0.05 versus control, AD and MSCs groups, respectively.
Changes in the signal transducer and activator of transcription (STAT3) protein expression in AD model brain tissue
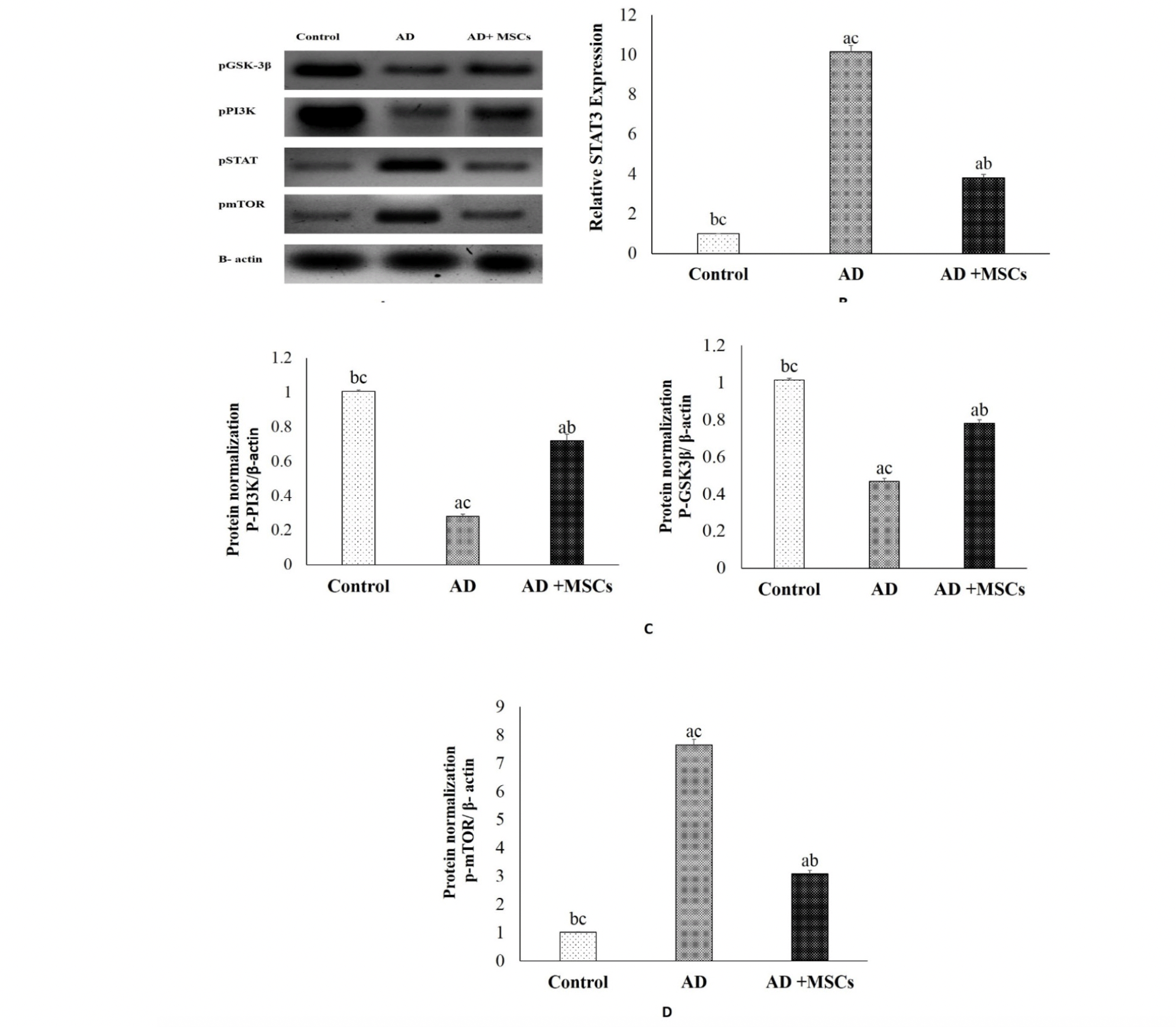
Based on the link between brain inflammation and the STAT3 pathway, our results revealed increased expression of STAT3 protein accompanied with higher levels of IL-1β in the brain of the AD model as shown in figure (4 A&B). However, treatment with MSCs suppresses the STAT3 expression due to the decrement of the pro-inflammatory cytokines. These results suggest that MSCs promote neurogenesis via their anti-inflammatory effect.
(A) Quantitative western blotting analysis (B) p STAT3, (C) p GSK-3β and p-PI3K, (D) mTOR proteins expression and quantification. Expression was normalized to β-actin as a loading control. Data are presented as the means ± SE (n= 6). a, b and c denote significant change at p<0.05 versus control, AD and MSCs groups, respectively.
Effects of MSCs on the PI3K/AKT/ GSK-3β signal pathway
The PI3K/AKT/ GSK-3β pathway is important for neuron survival. Its impairment is observed in many disorders, including psychiatric and neurological diseases, inflammatory diseases, and cancer. In this study, the phosphorylation levels of PI3K, and GSK-3β proteins in the brain tissues were detected by western blot. As shown in figure (4A&C), the expression of the p-PI3K and p-GSK-3β were markedly decreased in the brain of the AlCl3-treated rats, as compared to the control group. However, treatment with MSCs effectively ameliorated the downregulation of the p-PI3K as well as inhibited the activation of GSK-3β via increasing the expression of the p-GSK-3β. These results indicate that MSCs modulating the PI3K/GSK-3β signaling pathway.
Effects of MSCs on the autophagy
There is strong evidence that autophagy participates in the intracellular degradation of Aβ. Meanwhile, in the brain of AD patients, this mechanism is dysregulated. The current investigation revealed a significant upregulation of phosphorylated mTOR protein in the brain of the AD model (figure 4A&D). Meanwhile, MSCs transplantation downregulates the expression of mTOR mediated by Aβ-dependent excessive accumulation. Consequently, we hypothesized that MSCs inhibit mTOR and enhance autophagy and lysosomal clearance of Aβ.
Effects of MSCs on the SIRT1 and MiR-134 gene expression
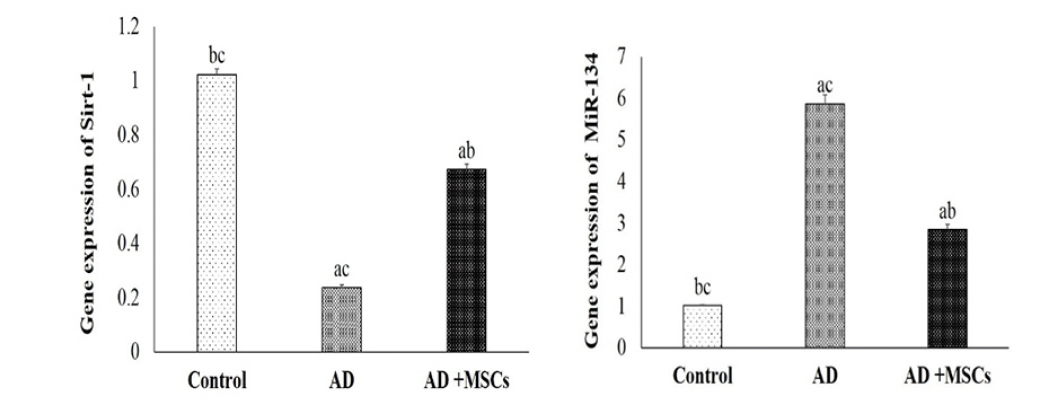
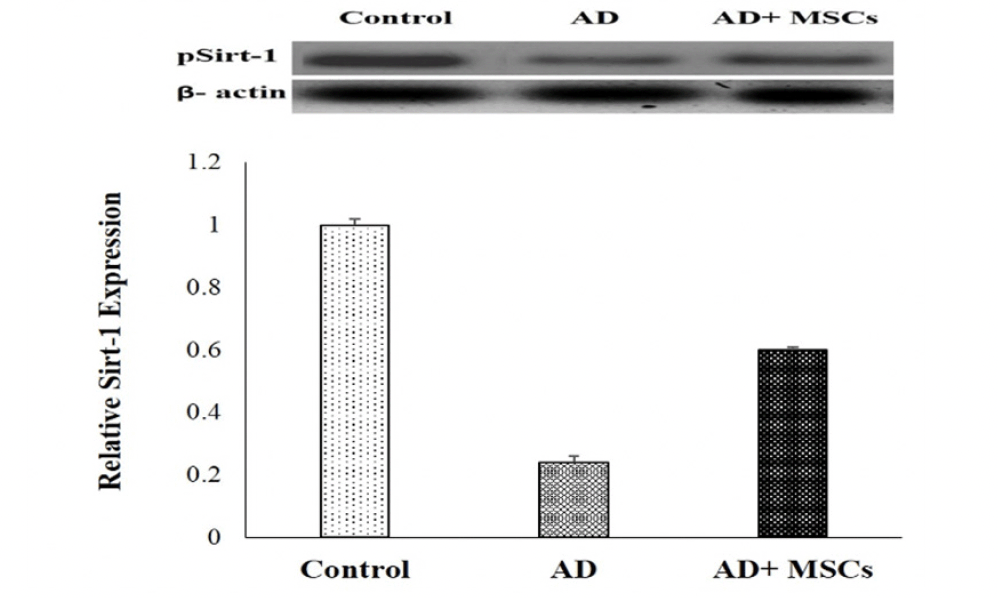
In the current study, the gene expression and the relative protein level of SIRT1 were significantly suppressed whereas the gene expression of the MiR-134 was increased in the brain tissues of AD compared to a control group. On the other hand, the intravenous injection of MSCs has markedly reversed these results via upregulation of SIRT1in gene and protein level and downregulation of MiR-134 gene expression (Figure 5 &6).
Data are presented as the means ± SE (n= 6). a, b and c denote significant change at p<0.05 versus control, AD and MSCs groups, respectively.
Expression was normalized to β-actin as a loading control. Data are presented as the means ± SE (n= 6). a, b and c denote significant change at p<0.05 versus control, AD and MSCs groups, respectively.
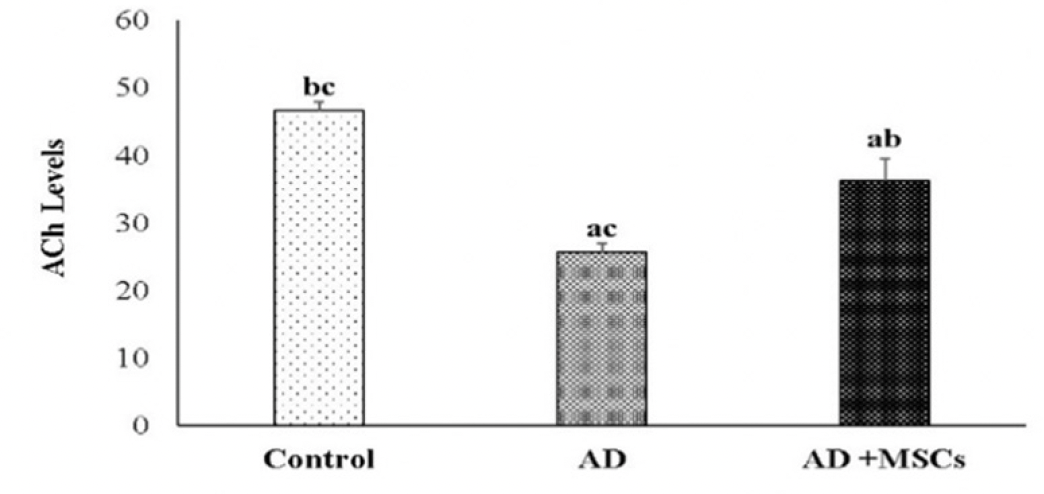
Effect of MSCs treatment on brain acetylcholine (ACh)
The obtained data showed impaired brain cholinergic neurotransmitters exhibited by a significant decrease in the ACh level in the brain of the AD rat model compared to control. Meanwhile, MSCs transplantation significantly increased ACh concentration in the brain as compared to the AD rat model (figure 7).
Data are presented as the means ± SE (n=6), a, b and c denote significant change at p<0.05 versus control, AD and MSCs groups, respectively.
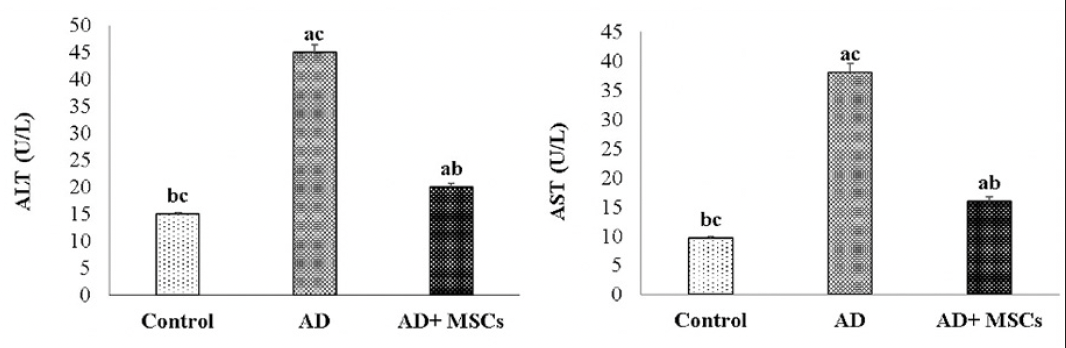
Effect of MSCs on hepatic dysfunction
The Aluminum toxicity touched liver tissues causing extraordinarily liver injury and impaired liver function accompanied by extensive leakage of the hepatic enzymes (ALT and AST) to the blood, thus increase their levels in the blood in response to administration with AlCl3. However, treatment with MSCs markedly attenuates the increment in these serum liver function parameters as illustrated in figure (8).
Data are presented as the means ± SE (n=6), a, b and c denote significant change at p<0.05 versus control, AD and MSCs groups, respectively.
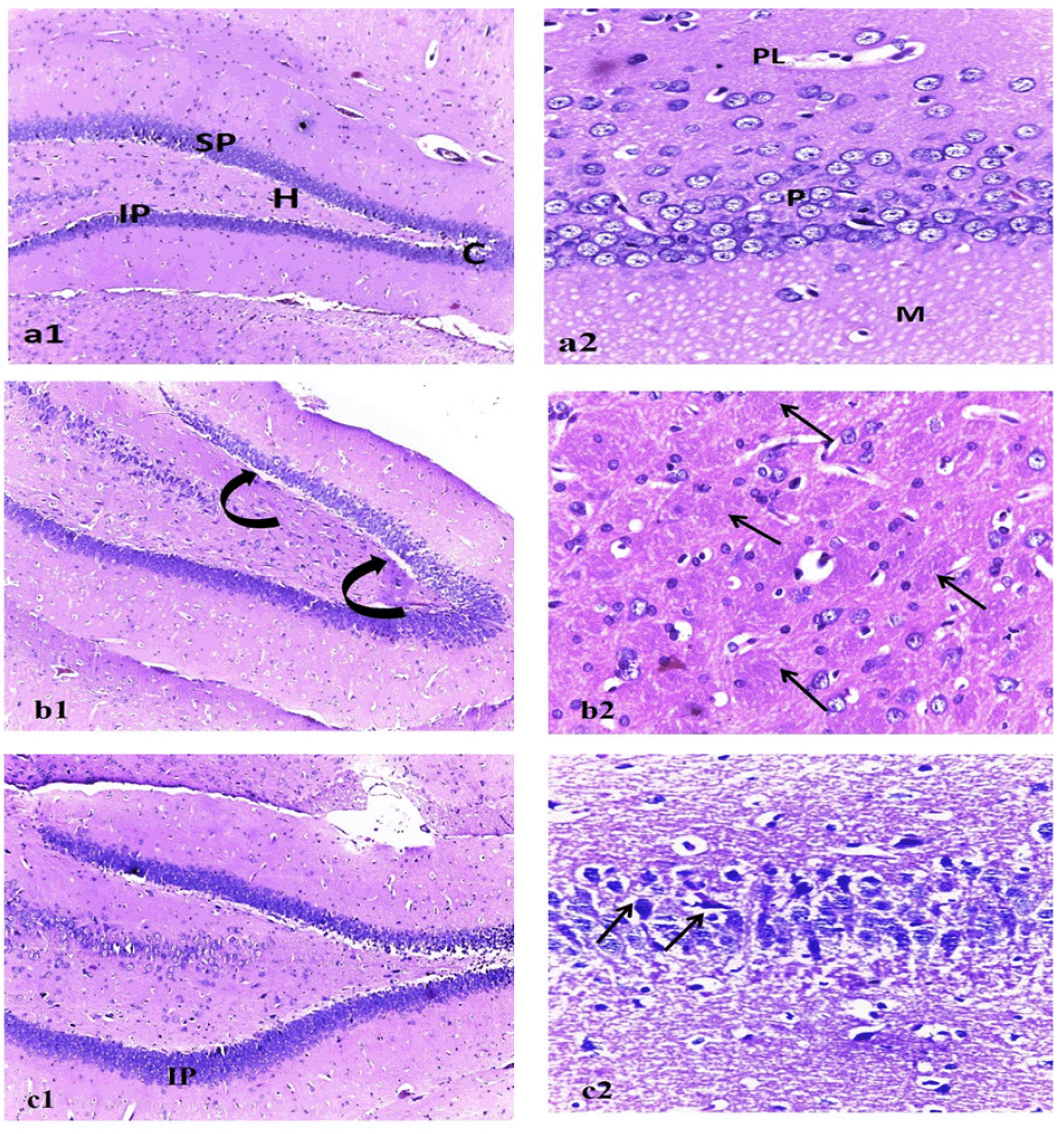
Histopathological investigation
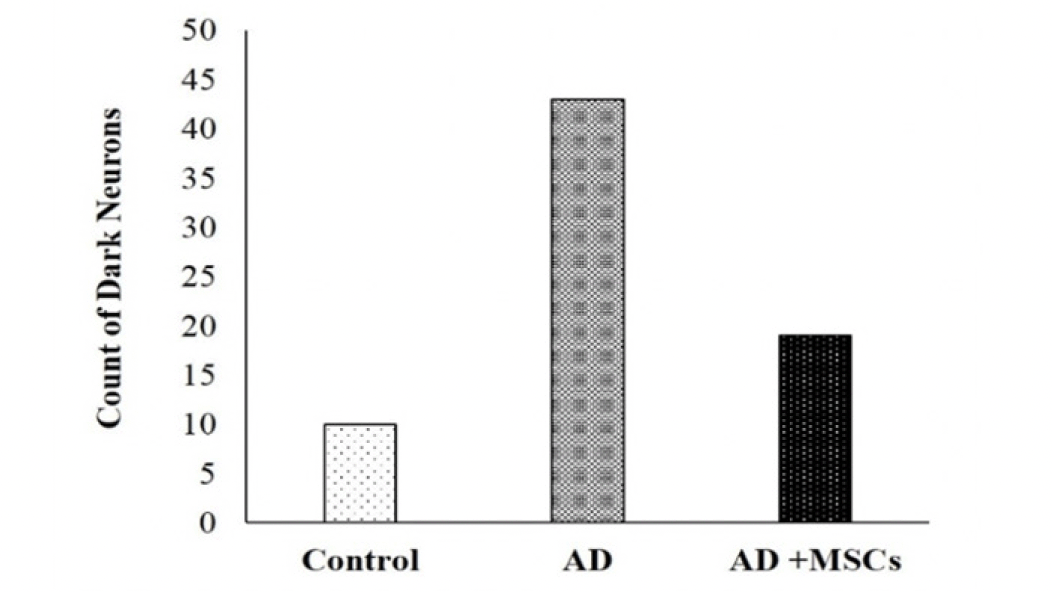
The photomicrograph of the brain tissue section of the control group showing a normal histological structure of the brain with normal neuronal morphology (hippocampus) figure (9, a1&a2). On the other hand, exposure to AlCl3 showed various sizes of multiple amyloid plaques formation in the hippocampus (Figure 9, b1). Moreover, abundant dark neurons (Figure 10), edema, hypoplasia, and neuronal degeneration were observed in figure (9, b2). Thus, confirming the neurodegeneration within the hippocampus as compared to the control group. Whereas, the sections of the AD group treated with MSCs showing (figure 9, c1) improvement of the CA1 region of hippocampus except for few neurons with peri-neuronal halos (arrows) accompanied with an increased neuronal density of the infra-pyramidal (IP) limbs of the dentate gyrus and decreased number of dark neurons (figure 9, c2).
Figure (a1) represents (control), showing normal dentate gyrus with normal limbs [suprapyramidal (SP) and infra-pyramidal (IP)], crest (C), and hilus (H). (a2) CA1 region of the hippocampus with healthy outer polymorphic layer (PL), middle pyramidal (P), and inner molecular (M) layers. Figure (b1): represent Alzheimer group showing dentate gyrus with vacuolations (curved arrows) invading the supra pyramidal limb. (b2) multiple cotton wool plaques (pathognomonic of alzheimer disease) (arrows). Figure (c1): represents the MSC-treated group showing increased neuronal density of the infra pyramidal (IP) limbs of the dentate gyrus. (c2) Improvement of the CA1 region of hippocampus except for few neurons with peri-neuronal halos (arrows). (1)H&E x100, (2) H&E x400.
Discussion
Aluminum (Al) hypothesis is the intoxication of the brain with Al through either environmental pollution, widespread use in daily life, or medications (2). The most affected brain regions are the hippocampus and frontal cortex, in which Alzheimer’s disease (AD) developed (31).
The pathogenesis of AD is a multifactorial neurodegenerative process with several damaged pathways due to oxidative stress injury, abnormal energy processing, mitochondrial dysfunction, and inflammation (8). Consequently, the induction of AD impaired several signal pathways, through the deposition of Aβ and p-tau protein besides oxidative stress. Therefore, the results revealed disturbance in the gene and protein expression levels of many signaling pathways involved in AD pathology, including mTOR, GSK-3β, and SIRT1/MiR-134.
In the present study, AD is characterized by a proteinopathy, which is an excessive accumulation of misfolded proteins like Aβ-42 and p-tau. Consistent with the earlier results of Fang et al. (32) this may be due to the dysfunction of proteasomes induces disturbance in the production and clearance of Aβ peptide together with hyperphosphorylated tau protein which loses its ability to bind to microtubules and eventually forming neurofibrillary tangles and microtubule disruption (33). Moreover, these results were confirmed by the histopathological appearance of multiple cotton wool plaques of the accumulated aggregates of Aβ in the CA1 of the hippocampus. These plaques caused neuronal dystrophy, and finally neuronal death (34) that is represented by the increased number of dark neurons in the AD group. In agreement with the finding of Duncan and Valenzuela (35), MSC transplantation showed a marked decline in the accumulated misfolded proteins (Aβ-42 and p-tau), thus inhibit Aβ- and tau-related cell death. Additionally, MSCs activated the enzymes responsible for amyloid β plaques degradation leading to reduction of cerebral amyloidosis (36). Moreover, Kim et al. (37) reported that MSC transplantation promotes clearance of abnormal Aβ plaques and tau-phosphorylation through differentiation to microglia or recruitment of activated microglia.
The high content of oxygen and polyunsaturated fatty acid along with low antioxidant levels make the brain vulnerable to oxidative stress and reactive oxygen species (ROS) (38). In AD, both Aβ and mitochondrial dysfunction are responsible for the production of ROS (39). Accordingly, the present study showed an impairment of the redox state in the AD brain accompanied by increased MDA level and abolished/ depleted brain antioxidant markers (catalase and glutathione) leading to neuronal damage. These results are in harmony with that of Lakshmi et al. (40). On the other hand, MSCs transplantation significantly attenuates the impaired redox state in the brain of the AD model, which may be due to scavenging ROS and enhancing the endogenous antioxidant agents in neurons. Our results are in line with that of Jiao et al. (41).
Misfolded and aggregated proteins bind to pattern recognition receptors on micro-and astroglia and trigger an innate immune response, characterized by the release of proinflammatory mediators (42). Furthermore, mitochondrial damage induced by Aβ triggers neuroinflammatory signals mediated via prolonged activation of microglia and astrocyte cells (43, 44). The present study showed a significant increase in the pro-inflammatory cytokines IL-1βand accompanied with upregulation of pSTAT3 in the brain of the AD model (45).
Additionally, Yang et al. (33) indicate the involvement of glycogen synthase kinase-3β (GSK-3β), a downstream kinase of the PI3K/Akt signaling pathway, constitutively active protein in neuroinflammation. Mechanistically, this can be attributed to increased GSK3 β activation that resulted from reduced PI3K-Akt-dependent GSK3 β phosphorylation (46). The obtained results revealed marked downregulated expression of the p-PI3K and p-GSK-3β in the brain of the AD model. Our data are in line with that of Yu and Koh (47) who reported that the inhibition of PI3K/AKT/ GSK-3β pathway was due to Aβ oligomers and oxidative stress in the neuron.
In contrast, treatment with MSCs reduced the release of the pro-inflammatory cytokines IL-1β and the expression of pSTAT3. The results of Han et al. (48) showed that exosomes and neurotrophic factors secreted from MSCs improve neuronal functions and pathological lesions in cortical brain tissue as well as attenuation of neuronal apoptosis through downregulation of p-STAT3. Choi et al. (49) as well showed that inhibition of STAT3 phosphorylation attenuates astrocytic function via converting the astrocytes from reactive into resting state, thus alleviate the inflammatory response. Furthermore, Park et al. (50) and Nakano et al. (51) showed that MSCs switch microglial phenotype from M1 pro-inflammatory to M2 anti-inflammatory (i.e. decrease the ratio of M1/M2 activated microglia). Therefore, MSCs ameliorate inflammation-induced neuronal cellular degeneration, reduce microgliosis, and prevent reactive astrogliosis (52). In addition to their immune-modulating and anti-inflammatory effects through the upregulation of neuroprotective and downregulation of pro-inflammatory cytokines (53).
Interestingly, autophagy is a catabolic process responsible for the degradation and recycling of macromolecules and organelles, therefore it has an important role in longevity through neuronal protection (54). Under normal conditions, the autophagic-lysosomal pathway cleared the Aβ aggregates (55). However, insufficient autophagy is associated with harmful protein aggregates, which results in increased ROS, cell death, and neurodegeneration (56). Consistent with our results Silva et al. (57) showed that upregulated mTOR signaling associated with AD may inhibit autophagy. Meanwhile, MSCs transplantation into an AD model resulted in a marked downregulation of the mTOR expression, therefore, enhance autophagy. In harmony with this, Shin et al. (58) showed that MSCs activate the autophagy-lysosome pathway, thereby enhance the clearance of neurotoxic Aβ deposits. Moreover, Nakano et al. (51) reported that treatment with BM-MSC up-regulated the number of M2 type of microglia, thus promote the clearance of the Aβ by phagocytosis, tissue repair, and secretion of anti-inflammatory cytokines. Therefore, improve neuronal activity by protecting from the toxicity of Aβ and tau (59). Consequently, this verifies that MSCs act as an autophagy modulator.
The current data showed that the gene expression of SIRT1was decreased whereas that of MiRNA-134 was upregulated in response to AD induction. Parallel to Yoshiyama et al. (60) SIRT1 deficiency is accompanied by higher levels of IL-1β resulting in synaptic loss and memory deficits associated with AD development. Additionally, Madadi et al. (61) reported that the dysregulation of miRNAs altered the balance between synthesis and clearance of Aβ peptide in AD. On the other hand, MSCs treatment significantly upregulates the expression of SIRT1.
Interestingly, through several pathways, SIRT1 may improve numerous hallmarks associated with the development of AD. Accordingly, SIRT1 activates PI3K/Akt, which in turn downregulated the phosphorylated form GSK3β therefore, increase neurite outgrowth, improve mitochondrial function and reduce the production of Aβ peptides and tau phosphorylation (62, 63). Moreover, upregulation of SIRT1 reverse the neuroinflammatory response by suppressing the sustained activation of microglia proliferation (64). Furthermore, SIRT1 enhances the autophagy-lysosomal pathway through direct de-acetylation of autophagy components (65) as well as downregulation and inhibition of mTOR signaling (66). Finally, SIRT1 directly promotes synapse plasticity and memory via a miR-134-mediated posttranscriptional mechanism. It cooperates with Yin Yang 1 (YY1) to limit the expression of miR-134 (62).
Chiroma et al. (67) indicated that the cholinergic system is involved in the memory retention process, therefore the cognitive impairments are accompanied by cholinergic dysfunctions (68). Our results showed a significant decrease in the level of the ACh. According to the finding of John et al. (69), the declined level of the ACh in the brain of the AD rat model was due to the cholinotoxic effect of AlCl3 via impairment of the synthesis and release process of this neurotransmitter, resulting in synaptic loss and memory deficits. In contrast, treatment with MSCs attenuated the reduced levels of acetylcholine (70). This may be due to the ability of MSCs to enhance endogenous neurogenesis that replaces damaged neurons in the AD brain, stimulate neuronal differentiation, and neuronal integration. These new neurons are capable of secreting neurotrophic factors and increasing brain ACh levels, thus improving neurocognitive functions in AD animal models (71). Qin et al. (72) demonstrated that MSCs transplantation markedly improves the cognitive deficits and alleviates neuropathology in the AD model via the suppression of apoptosis and the maintenance of functional synaptic contacts (73). Furthermore, it was found that BM-MSC treatment attenuated the cognitive impairment through the restoration of astrocytic function as well as suppression of the inflammation and promotion of synaptogenesis (51) besides their role in the upregulation of SIRT1 which promotes synapse plasticity and memory. This confirms the efficiency of MSCs in improving the cognitive deficits associated with the AlCl3-induced AD model in rats.
Conclusion
In conclusion, the neuroprotective potential of MSCs is verified through attenuation of the astrocytic inflammation via downregulating pro-inflammatory mediators and activation of microglial cells. MSCs enhance autophagy together with suppressing proteinopathies due to their anti-amyloidogenic potential. Enhancing neurogenesis to replace damaged neurons, improves both neuropathology and neurocognitive functions. Subsequently, MSCs transplantation could represent a promising and safe approach to treat aluminum-induced AD and its detrimental neurochemical changes. However, further studies are required to determine more mechanisms.
Acknowledgments: We appreciate the contribution of Prof. Dr. Basma Emad, professor of histology, Faculty of Medicine, Cairo University, for assistance in setting up histological examinations.
Author contributions: Omayma A.R. Abozaid designed the experimental study and revised the manuscript, provided technical support, Mohsen M. Sallam prepare materials, performed the methodology parts, collect the data, and wrote the part of the manuscript. Esraa S.A. Ahmed corrected the manuscript and contributed the materials and data analysis. All authors read and approved the final manuscript.
Funding: The authors did not receive support from any organization for the submitted work.
Availability of Data and Materials: All data are presented in the manuscript.
Ethics approval: The experimental protocol was carried out according to the Guide for the Care and Use of Laboratory Animals (NIH No. 85–23, 1996).
Competing Interests: The authors declare no competing interests
Open Access: This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
References
1. Igbokwe IO, Igwenagu E, Igbokwe NA. Aluminium toxicosis: a review of toxic actions and effects. Interdiscip Toxicol. 2019;12(2):45-70. doi: 10.2478/intox-2019-0007
2. Colomina MT, Peris-Sampedro F. Aluminum and Alzheimer’s Disease. Adv Neurobiol. 2017;18:183–97. DOI: 10.1007/978-3-319-60189-2_9
3. Okail HA, Ibrahim AS, and Badr AH. The protective effect of propolis against aluminum chloride-induced hepatorenal toxicity in albino rats. The Journal of Basic and Applied Zoology. (2020) 81:34 doi.org/10.1186/s41936-020-00169-9
4. Ogunlade B, Adelakun SA, Agie JA. Nutritional supplementation of gallic acid ameliorates Alzheimer-type hippocampal neurodegeneration and cognitive impairment induced by aluminum chloride exposure in adult Wistar rats. Drug Chem Toxicol. 2020;1-12. doi:10.1080/01480545.2020.175484.
5. Liu L, Liu Y, Zhao J, Xing X, Zhang C, Meng H. Neuroprotective Effects of D-(-)-Quinic Acid on Aluminum Chloride-Induced Dementia in Rats. Evidence-Based Complementary and Alternative Medicine Volume 2020, Article ID 5602597, 10 pages. doi.org/10.1155/2020/5602597
6. Kumar SEP, Bairy KL, Nayak V, Reddy S K, Kiran A, Ballal A. Amelioration of Aluminium Chloride (AlCl3) Induced Neurotoxicity by Combination of Rivastigmine and Memantine with Artesunate in Albino Wistar Rats. Biomed Pharmacol J 2019;12(2). DOI : https://dx.doi.org/10.13005/bpj/1692
7. Reitz C, Brayne C, Mayeux R. Epidemiology of Alzheimer’s disease. Nat Rev Neurol. 2011; 7(3):137-52. DOI: 10.1038/nrneurol.2011.2
8. Lin YT, Wu YC, Sun GC, et al. Effect of Resveratrol on Reactive Oxygen Species-Induced Cognitive Impairment in Rats with Angiotensin II-Induced Early Alzheimer’s Disease. J Clin Med. 2018; 5;7(10):329. DOI: 10.3390/jcm7100329
9. Dolati S, Yousefi M, Mahdipour M, Afrasiabi Rad A, Pishgahi A, Nouri M, et al. Mesenchymal stem cell and bone marrow mononuclear cell therapy for cardiomyopathy: from bench to bedside. J Cell Biochem. 2018; 120(1):45-55. DOI: 10.1002/jcb.27531
10. Vissers C, Ming GL, Song H. Nanoparticle technology and stem cell therapy team up against neurodegenerative disorders. Adv Drug Deliv Rev. 2019 Aug;148:239-251. DOI: 10.1016/j.addr.2019.02.007
11. Yao P, Zhou L, Zhu L, Zhou B, Yu Q. Mesenchymal Stem Cells: A Potential Therapeutic Strategy for Neurodegenerative Diseases. Eur Neurol. 2020; 83(3):235-241. DOI: 10.1159/000509268
12. Fu X, Liu G, Halim A, Ju Y, Luo Q, Song AG. Mesenchymal Stem Cell Migration and Tissue Repair. Cells. 2019 Jul 28;8(8):784. doi: 10.3390/cells8080784
13. Eom YW, Shim KY, Baik SK. Mesenchymal stem cell therapy for liver fibrosis. Korean J Intern Med. 2015;30(5):580-589. DOI:10.3904/kjim.2015.30.5.580
14. Kang SH, Kim MY, Eom YW, Baik SK. Mesenchymal Stem Cells for the Treatment of Liver Disease: Present and Perspectives. Gut Liver. 2020;14(3):306-315. doi:10.5009/gnl18412
15. Lee SH, Lee JH, Lee HY, Min KJ. Sirtuin signaling in cellular senescence and aging. BMB Rep. 2019 Jan;52(1):24-34. . DOI: 10.5483/BMBRep.2019.52.1.290
16. Wong SY, Tang BL. SIRT1 as a therapeutic target for Alzheimer’s disease. Rev Neurosci. 2016 Dec 1;27(8):813-825. DOI: 10.1515/revneuro-2016-0023
17. Gao J, Wang WY, Mao YW, Gräff J, Guan JS, Pan L, Mak G, Kim D, Su SC, Tsai LH. A novel pathway regulates memory and plasticity via SIRT1 and miR-134. Nature. 2010 Aug 26;466(7310):1105-9. DOI: 10.1038/nature09271
18. Maoz R, Garfinkel BP, Soreq H. Alzheimer’s Disease and ncRNAs. Adv Exp Med Biol. 2017;978:337-361. DOI: 10.1007/978-3-319-53889-1_18
19. Bielefeld P, Mooney C, Henshall DC, Fitzsimons CP. miRNA-Mediated Regulation of Adult Hippocampal Neurogenesis; Implications for Epilepsy. Brain Plast. 2017 Nov 9;3(1):43-59. DOI: 10.3233/BPL-160036
20. Liu, PP, Xie, Y, Meng, XY. et al. History and progress of hypotheses and clinical trials for Alzheimer’s disease. Sig Transduct Target Ther. 2019; 4, 29. DOI: 10.1038/s41392-019-0063-8
21. Hu Z, Li Z. miRNAs in synapse development and synaptic plasticity. Curr Opin Neurobiol. 2017;45:24-31. . doi: 10.1016/j.conb.2017.02.014
22. Alhadlaq A, Mao JJ. Mesenchymal stem cells: isolation and therapeutics. Stem Cells Dev. 2004 Aug;13(4):436-48. . DOI: 10.1089/scd.2004.13.436
23. Prockop DJ, Sekiya I, Colter DC. Isolation and characterization of rapidly self-renewing stem cells from cultures of human marrow stromal cells. Cytotherapy. 2001;3(5):393-6. DOI: 10.1080/146532401753277229
24. Justin Thenmozhi A, William Raja TR, Manivasagam T, Janakiraman U, Essa MM. Hesperidin ameliorates cognitive dysfunction, oxidative stress and apoptosis against aluminium chloride induced rat model of Alzheimer’s disease. Nutr Neurosci. 2017 Jul;20(6):360-368. DOI: 10.1080/1028415X.2016.1144846
25. Yoshioka T, Kawada K, Shimada T, Mori M. Lipid peroxidation in maternal and cord blood and protective mechanism against activated-oxygen toxicity in the blood. Am J Obstet Gynecol. 1979 Oct 1;135(3):372-6. DOI: 10.1016/0002-9378(79)90708-7
26. Sinha AK. Colorimetric assay of catalase. Anal Biochem. 1972 Jun;47(2):389-94. DOI: 10.1016/0003-2697(72)90132-7
27. Beutler E, Duron O, Kelly BM. Improved method for the determination of blood glutathione. J Lab Clin Med. 1963 May;61:882-8.
28. Reitman S, Frankel S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am. J. Clin. Path. 1957; 28: 56-63. DOI: 10.1093/ajcp/28.1.56
29. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248-54. DOI: 10.1006/abio.1976.9999
30. Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001 May 1;29(9):e45. DOI: 10.1093/nar/29.9.e45
31. Kot, F.S. The Effect of Natural Geochemical Background on Neurological and Mental Health. Expo Health, 2020; 12, 569–591.
32. Fang Y, Ou S, Wu T, et al. Lycopene alleviates oxidative stress via the PI3K/Akt/Nrf2pathway in a cell model of Alzheimer’s disease. PeerJ. 2020;8:e9308. doi:10.7717/peerj.9308
33. Yang Wen, Yue Liu, Qing-Qing Xu, Yan-Fang Xian, Zhi-Xiu Lin, “Sulforaphene Ameliorates Neuroinflammation and Hyperphosphorylated Tau Protein via Regulating the PI3K/Akt/GSK-3β Pathway in Experimental Models of Alzheimer’s Disease”, Oxidative Medicine and Cellular Longevity, vol. 2020, ArticleID 4754195, 17 pages, 2020. doi.org/10.1155/2020/4754195
34. El Hajj H, Savage JC, Bisht K et al. Ultrastructural evidence of microglial heterogeneity in Alzheimer’s disease amyloid pathology. J Neuroinflammation 16, 87 (2019). doi.org/10.1186/s12974-019-1473-9
35. Duncan T, Valenzuela M. Alzheimer’s disease, dementia, and stem cell therapy. Stem Cell Res Ther. 2017 May 12;8(1):111. DOI: 10.1186/s13287-017-0567-5
36. Harach T, Jammes F, Muller C, et al. Administrations of human adult ischemia-tolerant mesenchymal stem cells and factors reduce amyloid beta pathology in a mouse model of Alzheimer’s disease. Neurobiol Aging. 2017;51:83-96. doi:10.1016/j.neurobiolaging.2016.11.009
37. Kim KS, Kim HS, Park JM, Kim HW, Park MK, Lee HS, Lim DS, Lee TH, Chopp M, Moon J. Long-term immunomodulatory effect of amniotic stem cells in an Alzheimer’s disease model. Neurobiol Aging. 2013 Oct; 34(10):2408-20). DOI: 10.1016/j.neurobiolaging.2013.03.029
38. Li J, O W, Li W, Jiang ZG, Ghanbari HA. Oxidative stress and neurodegenerative disorders. Int J Mol Sci. 2013 Dec 16;14(12):24438-75. DOI: 10.3390/ijms141224438
39. Yokokawa K, Iwahara N, Hisahara S, et al. Transplantation of Mesenchymal Stem Cells Improves Amyloid-β Pathology by Modifying Microglial Function and Suppressing Oxidative Stress. J Alzheimers Dis. 2019;72(3):867-884. DOI: 10.3233/JAD-190817
40. Lakshmi BV, Sudhakar M, Prakash KS. Protective effect of selenium against aluminum chloride-induced Alzheimer’s disease: behavioral and biochemical alterations in rats. Biol Trace Elem Res. 2015 May;165(1):67-74. DOI: 10.1007/s12011-015-0229-3
41. Jiao H, Shi K, Zhang W, et al. Therapeutic potential of human amniotic membrane-derived mesenchymal stem cells in APP transgenic mice. Oncol Lett. 2016;12(3):1877-1883. doi: 10.3892/ol.2016.4857
42. Heneka MT, Carson MJ, El Khoury J, et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015;14(4):388-405. DOI: 10.1016/S1474-4422(15)70016-5
43. Sarlus H, Heneka MT. Microglia in Alzheimer’s disease. J Clin Invest. 2017 Sep 1;127(9):3240-3249. DOI: 10.1172/JCI90606
44. Tafoya MA, Madi S, Sillerud LO. Superparamagnetic nanoparticle-enhanced MRI of Alzheimer’s disease plaques and activated microglia in 3X transgenic mouse brains: Contrast optimization. J Magn Reson Imaging. 2017 Aug;46(2):574-588. DOI: 10.1002/jmri.25563
45. Wang Wen-Ying, Meng-Shan Tan, Jin-Tai Yu, Lan Tan., 2015. Role of pro-inflammatory cytokines released from microglia in Alzheimer’s disease. Ann Transl Med; 3(10):136. DOI: 10.3978/j.issn.2305-5839.2015.03.49
46. Hoffmeister L, Diekmann M, Brand K, Huber R. GSK3: A Kinase Balancing Promotion and Resolution of Inflammation. Cells. 2020;9(4):820. doi: 10.3390/cells9040820
47. Hyun-Jung Yu, Seong-Ho Koh. The role of PI3K/AKT pathway and its therapeutic possibility in Alzheimer’s disease. Hanyang Med Rev. 2017; 37:18-24. doi. org/10.7599/hmr.2017.37.1.18
48. Han M, Cao Y, Xue H, et al. Neuroprotective Effect of Mesenchymal Stromal Cell-Derived Extracellular Vesicles Against Cerebral Ischemia-Reperfusion-Induced Neural Functional Injury: A Pivotal Role for AMPK and JAK2/STAT3/NF-κB Signaling Pathway Modulation. Drug Des Devel Ther. 2020;14:2865-2876. Published 2020 Jul 20. doi:10.2147/DDDT.S248892
49. Choi M, Kim H, Yang EJ, Kim HS. Inhibition of STAT3 phosphorylation attenuates impairments in learning and memory in 5XFAD mice, an animal model of Alzheimer’s disease. J Pharmacol Sci. 2020 Aug;143(4):290-299. DOI: 10.1016/j.jphs.2020.05.009
50. Park HJ, Oh SH, Kim HN, Jung YJ, Lee PH. Mesenchymal stem cells enhance α-synuclein clearance via M2 microglia polarization in experimental and human parkinsonian disorder. Acta Neuropathol. 2016 Nov;132(5):685-701. DOI: 10.1007/s00401-016-1605-6
51. Nakano M, Kenta Kubota K, Kobayashi E, Chikenji TS, Yuki Saito Y, Konari N, Fujimiya M. Bone marrow derived mesenchymal stem cells improve cognitive impairment in an Alzheimer’s disease model by increasing the expression of microRNA 146a in hippocampus. Sci Rep.2020; 10:10772. doi.org/10.1038/s41598-020-67460-1
52. Drommelschmidt K, Serdar M, Bendix I, et al. Mesenchymal stem cell-derived extracellular vesicles ameliorate inflammation-induced preterm brain injury. Brain Behav Immun. 2017;60:220-232. doi:10.1016/j.bbi.2016.11.011
53. Vasic V, Barth K, Schmidt MHH. Neurodegeneration and Neuro-Regeneration-Alzheimer’s Disease and Stem Cell Therapy. Int J Mol Sci. 2019 Aug 31;20(17):4272. DOI: 10.3390/ijms20174272
54. Deng H, Mi MT. Resveratrol Attenuates Aβ25-35 Caused Neurotoxicity by Inducing Autophagy Through the TyrRS-PARP1-SIRT1 Signaling Pathway. Neurochem Res. 2016 Sep;41(9):2367-79. DOI: 10.1007/s11064-016-1950-9
55. Li L, Zhang S, Zhang X, Li T, Tang Y, Liu H, Yang W, Le W. Autophagy enhancer carbamazepine alleviates memory deficits and cerebral amyloid-β pathology in a mouse model of Alzheimer’s disease. Curr Alzheimer Res. 2013 May 1;10(4):433-41. DOI: 10.2174/1567205011310040008
56. Kou X, Chen D, Chen N. Physical Activity Alleviates Cognitive Dysfunction of Alzheimer’s Disease through Regulating the mTOR Signaling Pathway. Int J Mol Sci. 2019;20(7):1591. doi: 10.3390/ijms20071591
57. Silva JM, Rodrigues S, Sampaio-Marques B, Gomes P, Neves-Carvalho A, Dioli C, Soares-Cunha C, Mazuik BF, Takashima A, Ludovico P, Wolozin B, Sousa N, Sotiropoulos I. Dysregulation of autophagy and stress granule-related proteins in stress-driven Tau pathology. Cell Death Differ. 2019 Aug;26(8):1411-1427. DOI: 10.1038/s41418-018-0217-1
58. Shin JY, Park HJ, Kim HN, Oh SH, Bae JS, Ha HJ, Lee PH. Mesenchymal stem cells enhance autophagy and increase β-amyloid clearance in Alzheimer disease models. Autophagy. 2014 Jan;10(1):32-44. doi: 10.4161/auto.26508
59. Kobayashi E, Nakano M, Kubota K, et al. Activated forms of astrocytes with higher GLT-1 expression are associated with cognitive normal subjects with Alzheimer pathology in human brain. Sci Rep. 2018;8(1):1712. Published 2018 Jan 26. doi:10.1038/s41598-018-19442-7
60. Yoshiyama Y, Higuchi M, Zhang B, Huang SM, Iwata N, Saido TC, Maeda J, Suhara T, Trojanowski JQ, Lee VM. Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron. 2007 Feb 1;53(3):337-51. DOI: 10.1016/j.neuron.2007.01.010
61. Madadi S, Schwarzenbach H, Saidijam M et al. Potential microRNA-related targets in clearance pathways of amyloid-β: novel therapeutic approach for the treatment of Alzheimer’s disease. Cell Biosci. 2019; 9, 91. doi.org/10.1186/s13578-019-0354-3
62. Cao Y, Yan Z, Zhou T, Wang G. SIRT1 Regulates Cognitive Performance and Ability of Learning and Memory in Diabetic and Nondiabetic Models. J Diabetes Res. 2017;2017:7121827. DOI: 10.1155/2017/7121827
63. Villemagne VL, Doré V, Bourgeat P, Burnham SC, Laws S, Salvado O, Masters CL, Rowe CC. Aβ-amyloid and Tau Imaging in Dementia. Semin Nucl Med. 2017 Jan;47(1):75-88. DOI: 10.1053/j.semnuclmed.2016.09.006
64. Cho SH, Chen JA, Sayed F, et al. SIRT1 deficiency in microglia contributes to cognitive decline in aging and neurodegeneration via epigenetic regulation of IL-1β. J Neurosci. 2015;35(2):807-818. DOI: 10.1523/JNEUROSCI.2939-14.2015
65. Lee IH, Cao L, Mostoslavsky R, Lombard DB, Liu J, Bruns NE, Tsokos M, Alt FW, Finkel T. A role for the NAD-dependent deacetylase Sirt1 in the regulation of autophagy. Proc Natl Acad Sci U S A. 2008 Mar 4;105(9):3374-9. DOI: 10.1073/pnas.0712145105
66. Guo W, Qian L, Zhang J, Zhang W, Morrison A, Hayes P, Wilson S, Chen T, Zhao J. Sirt1 overexpression in neurons promotes neurite outgrowth and cell survival through inhibition of the mTOR signaling. J Neurosci Res. 2011 Nov;89(11):1723-36. DOI: 10.1002/jnr.22725
67. Chiroma SM, Mohd Moklas MA, Mat Taib CN, Baharuldin MTH, Amon Z. d-galactose and aluminium chloride induced rat model with cognitive impairments. Biomed Pharmacother. 2018;103:1602-1608. DOI:10.1016/j.biopha.2018.04.152
68. Bensalem J, Dal-Pan A, Gillard E, Calon F, Pallet V. Protective effects of berry polyphenols against age-related cognitive impairment, Nutr. Aging.2016; 3:89–106, DOI: 10.3233/NUA-150051
69. John J, Nampoothiri M, Kumar N, Mudgal J, Nampurath GK, Chamallamudi MR. Sesamol, a lipid lowering agent, ameliorates aluminium chloride induced behavioral and biochemical alterations in rats. Pharmacogn Mag. 2015;11(42):327-336. doi:10.4103/0973-1296.153086
70. Ma T, Gong K, Ao Q, et al. Intracerebral transplantation of adipose-derived mesenchymal stem cells alternatively activates microglia and ameliorates neuropathological deficits in Alzheimer’s disease mice. Cell Transplant. 2013;22 Suppl 1:S113-26. doi:10.3727/096368913X672181
71. Park D, Yang YH, Bae DK, et al. Improvement of cognitive function and physical activity of aging mice by human neural stem cells over-expressing choline acetyltransferase. Neurobiol Aging. 2013;34(11):2639-2646. doi:10.1016/j.neurobiolaging.2013.04.026
72. Qin, C., Lu, Y., Wang, K. et al. Transplantation of bone marrow mesenchymal stem cells improves cognitive deficits and alleviates neuropathology in animal models of Alzheimer’s disease: a meta-analytic review on potential mechanisms. Transl Neurodegener 9, 20 (2020). https://doi.org/10.1186/s40035-020-00199-x
73. Lee M, Ban JJ, Yang S, Im W, Kim M. The exosome of adipose-derived stem cells reduces β-amyloid pathology and apoptosis of neuronal cells derived from the transgenic mouse model of Alzheimer’s disease. Brain Res. 2018;1691:87-93. doi:10.1016/j.brainres.2018.03.034