C. Fompeyrine1,2, L.A. Abderhalden2, N. Mantegazza2, N. Hofstetter2, G. Bieri-Brüning3, H.A. Bischoff-Ferrari1,2,4, M. Gagesch1,2
1. Department of Geriatrics and Aging Research, University Hospital Zurich, Zurich, Switzerland; 2. Centre on Aging and Mobility, University of Zurich, Zurich, Switzerland
3. Zurich Geriatric Services and Nursing Homes, Zurich, Switzerland; 4. University Clinic for Acute Geriatric Care, City Hospital Waid and Triemli, Zurich, Switzerland.
Corresponding author: Michael Gagesch, MD, Dept. of Geriatrics and Aging Research, University Hospital Zurich, Raemistrasse 100, 8091 Zurich, Switzerland, michael.gagesch@usz.ch
J Frailty Aging 2020;in press
Published online October 20, 2020, http://dx.doi.org/10.14283/jfa.2020.58
Abstract
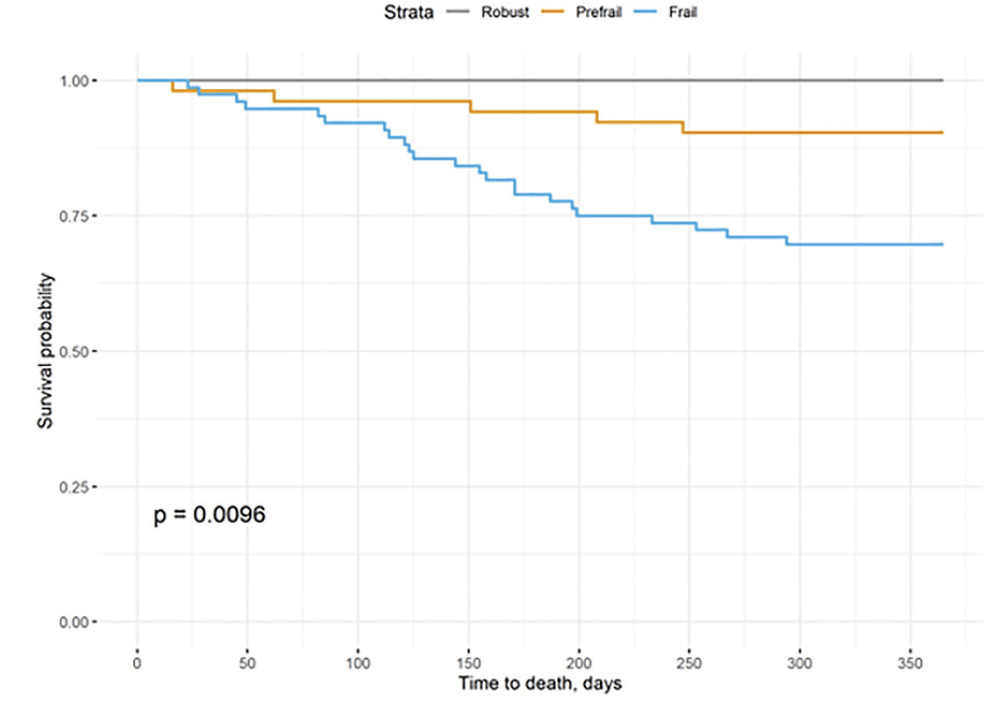
Frail older adults with ongoing care needs often require post-acute care (PAC) following acute hospitalization when not eligible for specific rehabilitation. Long-term outcomes of PAC in this patient group have not been reported for Switzerland so far. In the present report, we investigated 12-month mortality in regard to frailty status upon admission to PAC in a nursing home setting. In our sample of 140 patients (mean age 84 [±8.6] years) 4.3% were robust, 37.1% were pre-frail, 54.3% were frail and 4.3% were missing frailty status. Mortality at 12-months follow-up stratified by baseline frailty was 0% (robust), 11.5% (pre-frail) and 31.6% (frail). Kaplan-Meier analysis stratified by frailty status showed a decreased probability of 12-months survival for frail individuals compared to their pre-frail and robust counterparts (P = 0.0096). Being frail was associated with more than 4-fold increased odds of death at follow-up (OR 4.19; 95% CI 1.53-11.47).
Key words: Frailty, long-term mortality, post-acute care, nursing homes.
Introduction
Health in older age comprises a broad spectrum from robustness to vulnerability and frailty (1). The latter is associated with multiple negative outcomes in various care settings, from general practice to acute hospital care (2). With their often complex health status, older adult patients frequently require longer lengths of stay in the hospital and often remain at an increased level of care, impeding a prompt discharge home after an acute illness (3). At the same time, standard rehabilitation programs do not always appear suitable for many frail older patients (4).
Post-acute care (PAC) programs aim to bridge the gap between acute care and returning home for older adults, not otherwise eligible for rehabilitation. While earlier studies from different countries and specific settings (i.e. heart-failure patients) have demonstrated positive effects of PAC, such as reduced readmissions and decreased mortality rates (5, 6), its potential benefits and outcomes, particularly in frail older adults are still understudied (7). In addition, healthcare systems and PAC programs appear to have major differences between countries, hampering direct comparisons (8).
In a prior analysis, clinically significant improvements of physical function and ADL were reported in robust and frail Swiss older adults after a PAC program in nursing homes with a mean duration of 31 days (9). However, no research on the long-term outcomes of PAC in Swiss nursing homes exists so far (4). Therefore, the aim of our study was to investigate the association of frailty status upon admission to PAC with 12-month mortality in a real-world sample of Swiss older adults.
Methods
Study Design
We conducted a one-year follow-up study at designated PAC units of three municipal nursing homes within the City of Zurich, Switzerland. Written informed consent was obtained before study enrolment. The competent ethics committee of the Canton of Zurich approved our study (BASEC 2016-01069).
PAC Setting and Patients
Our study recruited consecutive patients 60 years and older referred to a PAC unit after acute care hospitalization between August and September 2016. An interdisciplinary team under the supervision of a board-certified geriatrician completed a comprehensive geriatric assessment (CGA) for each patient within one week upon admission, performed the PAC program and held bi-weekly team meetings. PAC consisted of activating nursing care (i.e. goal-directed instruction and training of ADL), five sessions of individual physical therapy per week and additional occupational therapy as needed, based upon the initial CGA. The maximum length of stay at PAC units was usually limited to 10 weeks duration and the effective date of discharge was based on the accomplishment of specific goals, derived from the individual care plan (9).
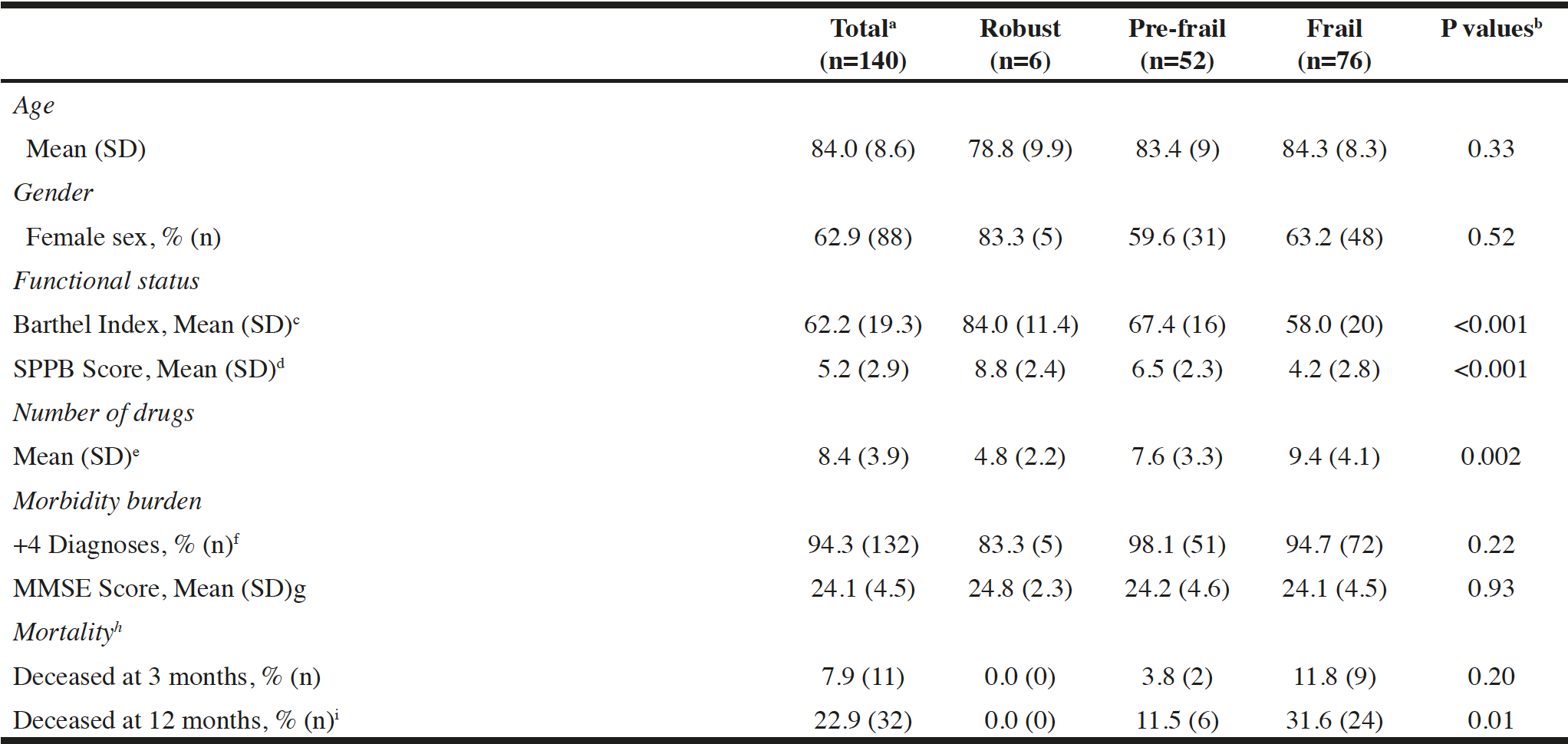
For our follow-up investigation, we matched the initial list of PAC patients with the death registry of the City of Zurich at one year after discharge. Living status and mortality date (if applicable) was recorded. We utilized frailty status from CGA at admission to a PAC unit according to the Fried frailty phenotype (items: unintentional weight loss, fatigue, slowness, weakness, low activity level) (10). Among numerous proposed frailty definitions, the Fried frailty phenotype is one of the most recognized and highly cited concepts and has been validated in various healthcare settings (2, 11). Patients with zero positive criteria were classified as robust, patients with 1-2 positive criteria as pre-frail and patients with ≥3 positive criteria were considered frail (10). In addition, we utilized further patient characteristics recorded at admission (Barthel-Index, Short physical performance battery (SPPB), Mini-Mental State Examination (MMSE) score, number of drugs and number of diagnoses) to describe the functional status and comorbidity burden.
Statistical Analysis
Three months and one year mortality rate after PAC discharge as well as further patient characteristics recorded at admission were calculated and stratified by level of frailty (robust, pre-frail, frail). Kaplan-Meier curves for visual representation were constructed for the overall sample to compare frail vs. robust and pre-frail at admission to PAC. Fisher’s exact test was used to evaluate whether mortality rate one year after discharge from PAC was independent of frailty status at admission. ANOVA and Chi-square test were used to evaluate whether there was a difference in mortality rate between frailty levels, as well as age and gender. Furthermore, a logistic regression model predicting mortality was evaluated to determine a possible association between frailty status upon admission to PAC and mortality rate on follow-up. The model was adjusted for age and gender. Statistical significance was determined as P<0.05 using 2-sided tests. All statistical analyses were performed using R v3.5.0 (The R Foundation for Statistical Computing, Vienna, Austria) and SAS v9.4 (SAS Institute, Inc. Cary, USA).
Results
Baseline Population
Our baseline sample consisted of n=140 patients, including 62.9% (n=88) women. Mean age at admission to PAC was 84 years (± 8.57). Mean length of stay at PAC was 31 days (± 16.5). In all, the most frequent diagnoses on admission to PAC were fractures (n=29), infections except pulmonary manifestations (n=18), mobility disorders (n=17), cognitive impairment (n=15), and heart disease (n=11), as reported earlier (12).
Mortality and Frailty Status
For n=139 patients, mortality status and mortality date at 3 and 12 months after discharge from PAC were applicable. At admission to PAC, 4.3% (n=6) of patients were robust, 37.1% (n=52) were pre-frail, 54.3% (n=76) were frail and 4.3% (n=6) were missing information on frailty status. The one-year mortality rate for the overall sample was 22.9% (32/140). One-year mortality rate stratified according to the different levels of frailty was 0% (robust, 0/6), 11.5% (pre-frail, 6/52) and 31.6% (frail, 24/76). Frailty status in relation to mortality, functional status and comorbidity burden is summarized in Table 1.
a. n=6 missing frailty status at admission; b. testing the difference between frailty levels; c. n=5 missing patients; d. SPPB, Short physical performance battery, n=10 missing patients; e. n=3 missing patients; f. n=1 missing patients; g. MMSE, Mini-Mental State Examination, n=7 missing patients; h. n=1 missing patient; i. n=2 deceased patients were missing frailty status at admission
For further analysis, we combined the group of robust and pre-frail patients, as none of the robust group deceased in the year following discharge from PAC. Our logistic regression model showed significantly increased odds of death for being frail (OR 4.19; 95% CI 1.53-11.47), and male gender (OR 3.19; 95% CI 1.28-8.0), but not for older age (OR 1.06, 95% CI 1.00-1.13 for each additional year).
Estimating survival with a Kaplan Meier analysis stratified by frailty status at admission to PAC showed a decreased probability of one-year survival for frail individuals, compared to patients classified as pre-frail or robust (P = 0.0096), Figure 1. In addition, each point increment on the frailty score at admission to PAC was associated with a decreasing one-year survival (P = 0.014).
Discussion
With more than one in two patients being frail and more than one in three being at risk for the condition (i.e. pre-frail) in our sample, frailty appears to be highly prevalent in Swiss older adults undergoing PAC in a nursing home setting. In comparison, the estimated prevalence of frailty in community-dwelling older adults in Switzerland is 5.8% (13). In our analysis, male gender and prevalent frailty were significantly associated with decreased survival at 12 months follow-up. In particular, frail patients had a greater than 4-fold increased odds for long-term mortality compared to their robust and pre-frail counterparts.
Our findings are in line with results from a prior study in older adults from Spain, where age, male gender and worse functional status were associated with higher 12-month mortality after acute illness (14). Our overall mortality rate of 22.9% is comparable to reports from earlier studies in former hospitalized geriatric patients from Germany and Italy (20.3% in Ritt et al. (15); 24.9% in Pilotto et al. (16)). However, those studies investigated one-year mortality after acute hospitalization without reporting on the utilization of PAC. Notably, patients in one of the aforementioned studies had a lower frailty prevalence at admission to acute care than our patient group (e.g. 43.3% vs. 54.3% in this study) (15).
When comparing the 12-month mortality rate of 31.6% in our frail patients with the aforementioned studies from acute care settings in Germany and Italy, it appears consistent with those reported by Ritt et al. (36.1%) and Pilotto et al. (24.9%) (15, 16). Of note, the higher mean age of patients in our study was more comparable to the first study (mean age >80 years), while Pilotto and colleagues investigated a sample with a mean age <80 years. Therefore, this difference is probably due to the influence of age in relation to the difference in mortality and warrants further investigation.
As a strength, our study is the first to report on the long-term outcomes of PAC in Swiss nursing homes and its association with frailty status. Further, we used a standardized operationalization of the Fried frailty phenotype, a derivation of the original version by Fried et al. (10). Our study also has its limitations. First, our sample size and short duration of patient recruitment limit the generalizability of our results. We also lack information on causes of death during follow-up. Furthermore, we had to cluster robust and pre-frail patients for our analysis, which might hinder comparisons to other studies. In addition, the frailty phenotype may not be the best frailty instrument to predict 12-month mortality in this patient group (15). Finally, our study did not include a control group of “standard” nursing home care residents to compare with our results regarding potential recovery time in the absence of specific interventions.
Conclusion
Our study in 140 former geriatric inpatients 12 months after discharge from PAC suggests that male gender and frailty status upon admission to PAC are significantly associated with increased long-term mortality in this group of Swiss older adults. While in line with prior studies from other populations, our study adds important knowledge on the specific situation in Switzerland. More studies are needed to further investigate the impact of PAC programs on short and long-term outcomes in Switzerland, including older adults affected by frailty.
Acknowledgments: We like to thank Marion Thalmann and Thomas Tröster for performing the initial data collection. In addition we thank Dr. Wei Lang for his statistical advice. Furthermore, we would like to thank all involved staff members at the participating municipal nursing homes in the City of Zurich.
Conflicts of Interest: The authors declare no conflict of interest.
Ethical Standards: The authors declare that the study porcedures comply with current ethical standards for research involving human participants in Switzerland. The study protocol has been approved by the Cantonal Ethics Committee of the Canton of Zurich, Switzerland (BASEC 2016-01069);
Open Access: This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
References
1. Buchner DM, Wagner EH. Preventing frail health. Clin Geriatr Med. 1992;8(1):1-17.
2. Dent E, Kowal P, Hoogendijk EO. Frailty measurement in research and clinical practice: A review. Eur J Intern Med. 2016;31:3-10.
3. Covinsky KE, Palmer RM, Fortinsky RH, Counsell SR, Stewart AL, Kresevic D, et al. Loss of independence in activities of daily living in older adults hospitalized with medical illnesses: increased vulnerability with age. J Am Geriatr Soc. 2003;51(4):451-8.
4. Kone I, Zimmermann B, Wangmo T, Richner S, Weber M, Elger B. Hospital discharge of patients with ongoing care needs: a cross-sectional study using data from a city hospital under SwissDRG. Swiss medical weekly. 2018;148:w14575.
5. Feltner C, Jones CD, Cené CW, Zheng ZJ, Sueta CA, Coker-Schwimmer EJ, et al. Transitional care interventions to prevent readmissions for persons with heart failure: a systematic review and meta-analysis. Ann Intern Med. 2014;160(11):774-84.
6. Prvu Bettger J, Alexander KP, Dolor RJ, Olson DM, Kendrick AS, Wing L, et al. Transitional care after hospitalization for acute stroke or myocardial infarction: a systematic review. Ann Intern Med. 2012;157(6):407-16.
7. Roberts PS, Goud M, Aronow HU, Riggs RV. Frailty in a Post-Acute Care Population: A Scoping Review. PM & R : the journal of injury, function, and rehabilitation. 2018.
8. Abrahamsen J, Rozzini R, Boffelli S, Cassinadri A, Ranhoff A, Trabucchi M. Comparison of Italian and Norwegian postacute care settings for older patients in need of further treatment and rehabilitation after hospitalization. The Journal of Aging Research and Clinical Practice (JARCP). 2015.
9. Thalmann M, Troster T, Fischer K, Bieri-Bruning G, Patrick B, Bischoff-Ferrari HA, et al. Do older adults benefit from post-acute care following hospitalisation? A prospective cohort study at three Swiss nursing homes. Swiss medical weekly. 2020;150:w20198.
10. Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146-56.
11. Hoogendijk EO, Afilalo J, Ensrud KE, Kowal P, Onder G, Fried LP. Frailty: implications for clinical practice and public health. Lancet. 2019;394(10206):1365-75.
12. Tröster T, Thalmann M, Fischer K, Bieri-Brüning G, Beeler PE, Bischoff-Ferrari HA, et al. Frailty, underweight and impaired mobility are associated with institutionalisation after post-acute care. Swiss medical weekly. 2020;150:w20276.
13. Santos-Eggimann B, Cuenoud P, Spagnoli J, Junod J. Prevalence of frailty in middle-aged and older community-dwelling Europeans living in 10 countries. J Gerontol A Biol Sci Med Sci. 2009;64(6):675-81.
14. Baztan JJ, Galvez CP, Socorro A. Recovery of functional impairment after acute illness and mortality: one-year follow-up study. Gerontology. 2009;55(3):269-74.
15. Ritt M, Bollheimer LC, Sieber CC, Gaßmann KG. Prediction of one-year mortality by five different frailty instruments: A comparative study in hospitalized geriatric patients. Arch Gerontol Geriatr. 2016;66:66-72.
16. Pilotto A, Rengo F, Marchionni N, Sancarlo D, Fontana A, Panza F, et al. Comparing the prognostic accuracy for all-cause mortality of frailty instruments: a multicentre 1-year follow-up in hospitalized older patients. PLoS One. 2012;7(1):e29090.