M.N. Sabbagh1, M. Boada1, S. Borson3, P. Murali Doraiswamy4, B. Dubois5, J. Ingram6, A. Iwata7, A.P. Porsteinsson8, K.L. Possin9, G.D. Rabinovici9, B. Vellas10, S. Chao11, A. Vergallo12, H. Hampel12
1. Cleveland Clinic Lou Ruvo Center for Brain Health, Las Vegas, NV, USA; 2. Memory Clinic and Research Center of Fundació ACE, Institut Català de Neurociències Aplicades, Universitat Internacional de Catalunya (UIC), Barcelona, Spain; 3. University of Washington School of Medicine, Seattle, Washington, and Dementia Care Research and Consulting, Santa Ana, CA, USA; 4. Departments of Psychiatry and Medicine, Duke University School of Medicine, USA; 5. Institute of Memory and Alzheimer’s Disease (IM2A), Department of Neurology, Center of excellence of neurodegenerative disease (CoEN) and National Reference Center for Rare or Early Dementias Pitié-Salpêtrière Hospital, AP-HP, Boulevard de l’hôpital, Paris, France; 6. Seniors Lead Physician, Central East Region, Ontario and Founder and Medical Director of Kawartha Centre, Peterborough, Ontario, Canada; 7. Department of Neurology, The University of Tokyo Graduate School of Medicine, Tokyo, Japan; 8. Department of Psychiatry, University of Rochester School of Medicine and Dentistry, Rochester, NY, USA; 9. Department of Neurology, University of California San Francisco, Memory and Aging Center, San Francisco, USA; 10. Gérontopôle de Toulouse, Institut du Vieillissement, Centre Hospitalo-Universitaire de Toulouse, Toulouse, France; UMR INSERM, 1027 University of Toulouse III, Toulouse, France Faculté de médecine, Toulouse, France; 11. ClearView Healthcare Partners – Newton, MA, USA; 12.Global Medical Affairs, Neurology Business Group, Eisai Inc., Woodcliff Lake, New Jersey, USA
Corresponding Author: Marwan N. Sabbagh, Cleveland Clinic Lou Ruvo Center for Brain Health, Las Vegas, NV, USA, sabbagm@ccf.org; Tel.: (702) 483-6029; Fax: (702) 722-6584
J Prev Alz Dis 2020;3(7):171-178
Published online April 6, 2020, http://dx.doi.org/10.14283/jpad.2020.22
Abstract
Emerging digital tools have the potential to enable a new generation of qualitative and quantitative assessment of cognitive performance. Moreover, the ubiquity of consumer electronics, such as smartphones and tablets, can be harnessed to support large-scale self-assessed cognitive screening with benefit to healthcare systems and consumers. A wide variety of apps, wearables, and new digital technologies are either available or in development for the detection of mild cognitive impairment (MCI), a risk factor for dementia. Two categories of novel methodologies may be considered: passive technologies (which monitor a user’s behavior without active user input) and interactive assessments (which require active user input). Such examinations can be self-administered, supervised by a caregiver, or conducted by an informant at home or outside of a clinical setting. These direct-to-consumer tools have the potential to sidestep barriers associated with cognitive evaluation in primary care, thus improving access to cognitive assessments. Although direct-to-consumer cognitive assessment is associated with its own barriers, including test validation, user experience, and technological concerns, it is conceivable that these issues can be addressed so that a large-scale, self-assessed cognitive evaluation that would represent an initial cognitive screen may be feasible in the future.
Key words: Alzheimer’s disease, mild cognitive impairment, cognitive screening, digital consumer.
Introduction
The incidence of age-related diseases such as Alzheimer’s disease (AD) will increase dramatically as the expected life-expectancy does. The increasing incidence of AD will fundamentally overburden healthcare institutions and services worldwide. The exponential growth of AD represents a major impact with severe medical, financial, ethical, emotional, and physical implications at both the individual and societal level. However, reasonable hope exists that disease-modifying therapies currently in late-stage clinical development can be approved in the near future. These candidate treatments target patients in early clinical stages of AD (i.e., mild cognitive impairment [MCI]) before dementia symptoms manifest and have the potential to significantly delay disease progression. However, healthcare systems need structural and functional innovation towards early detection and diagnosis of AD from as early as the preclinical/prodromal stages, in order to implement disease-modifying treatments once they will be available.
This article represents the third part of a three-part series of an expert consensus perspective on the screening, identification, and management of MCI, the barriers and frictions that prevent large-scale cognitive evaluation, and recommendations for test-makers going forward. These recommendations were delivered by a global panel of clinical and research experts focused on MCI and AD. The views and recommendations presented here represent the consensus opinion of this group based on meetings of the expert group in April 2019. The first article of this series of three publications covers more detail on the need for and value of testing for MCI as well as a review of existing consensus statements and recommendations in the literature on MCI clinical identification. The second article examines the current state of MCI testing in the primary care setting, key hurdles, and recommendations for future improvements. As described in the prior articles, we utilize the term “MCI” to refer to the broad definition of intermediate cognitive impairment due to a variety of etiologies and use the term “MCI due to AD” or “MCI-AD” to refer specifically to the MCI clinical syndrome associated with positive biomarkers of AD pathophysiology (1, 2). This article will focus on direct-to-consumer detection of cognitive impairment, intended to be conducted by an individual or their family member, outside of a physician’s office, such as at home or a retail pharmacy, without direct supervision by healthcare providers.
While evaluating cognitive performance outside of a clinical setting is less straightforward than traditional neuropsychometric evaluation in a clinical setting, the working group believes that the potential to leverage technology to monitor and understand cognitive function offers exciting possibilities. MCI screening has historically been considered strictly as a healthcare provider-administered assessment conducted in a clinical setting. Several companies have developed products and tools with an office-based clinical and commercial model in mind, incorporating per-use licensing fees with the expectation that the test could be reimbursed by insurance plans such as Medicare (3). However, for several reasons articulated in the second article of this series, these existing tools have not been widely adopted, prompting the need to consider alternative clinical and commercial approaches to not only office-based tools and assessments, but also innovative direct-to-consumer approaches focused on at-home use by individuals and informants. Technology-driven evaluation of cognitive performance is of great interest, given its potential to improve patient care, empower patients, and identify patients who are currently undetected through traditional healthcare avenues. At present, cognitive decline often remains undetected for a long time, ultimately forcing patients and families to cope with cognitive impairment and ensuing dementia and AD with little preparation or support. However, new digital technologies may rectify these gaps. For example, in the future, adults may be instructed to complete an online or telephone questionnaire at home prior to visiting their doctor. Two categories of novel methodologies may be considered: passive technologies that monitor user behavior without active user input and interactive assessments that require the adult user of these technologies to actively engage in the evaluation. For those individuals who proactively seek tools to monitor their own cognitive performance, smartphones use could be “passively” monitored for subtle changes suggestive of cognitive decline. Such functionality could even be incorporated into smart homes, such that changes in an individual’s activities of daily living can be detected and analyzed, alerting the patient or their physician that their cognitive or emotional state may have changed. Moreover, smart homes may offer the possibility of multi-dimensional and dynamic monitoring of cognitive performances, from simple to complex tasks.
Though home-based evaluation for MCI is associated with meaningful barriers, it will be critical to work toward making at-home testing accessible and scalable across a broad population to allow individuals with MCI-AD to present to a healthcare provider to initiate the diagnostic process, to eventually receive adequate care. It is important to note that the goal of at-home testing is not to replace current neuropsychometric testing and clinical evaluation by trained healthcare providers in the primary care setting, but to enable large-scale cognitive screening and to optimize access to cognitive-oriented care through a potentially more accurate and comprehensive evaluation pathway. Within this context, we have outlined current barriers that limit use and/or effectiveness of home-based cognitive evaluation and recommended potential solutions that may help overcome these parameters. We have also outlined parameters of an ideal home-based evaluation tool and provided an initial perspective on how home-based testing may be integrated with testing in a clinical setting, as a starting point for future investigation of the optimal care pathway for MCI individuals. Finally, we have briefly characterized recent developments in the field of direct-to-consumer or at-home cognitive performance evaluation and highlighted potentially disruptive (i.e., transformative for the current management paradigm) technologies in development.
Current landscape
Barriers Related to Test Validation
Currently, the quality of clinical data generated by at-home direct-to-consumer tests conducted by individuals and informants lags behind tests administered by healthcare providers in an office setting, some of which have decades of development and validation behind them (e.g., MMSE, MoCA, ADAS-Cog). Most direct-to-consumer tests and tools lack the robust clinical validation and data needed to create confidence that a test is accurate and repeatable across a heterogeneous test population. As with other clinical tests, at-home tools are unlikely to be approved by regulatory agencies for clinical use without validation in a controlled, clinical trial setting that includes study subjects representative of the diverse demographics of the real-world population. Lack of regulatory approval will result in difficulties with loss of credibility and reimbursement with patients and clinicians, further limiting the reach, availability, and impact of at-home tests. Investing in initial validation studies, robust implementation studies, and large-scale studies with long-term follow-up and a large sample size will be essential to legitimize the clinical value of at-home testing to physicians and, subsequently, to patients.
Barriers Related to User Experience
Potential users of direct-to-consumer cognitive tools may face a wide variety of potential barriers that prevent at-home cognitive evaluation. Test users may not be aware of any deficit in their cognitive function, particularly given that early dementia can be characterized by impaired self-awareness, or patients may be in denial that a cognitive concern exists for fear of the stigma associated with cognitive decline. In either case, the result is that many individuals will have little motivation to seek out and utilize at-home testing. Even if an individual or informant is concerned about a potential loss of cognitive function and motivated to act, they are unlikely to be aware of home-based assessments at their disposal. Additionally, even if a user completes a home-based test, these tests rarely provide actionable information about where to seek medical care given the lack of established care and referral pathways and lack of effective treatments for MCI due to AD. Another critical factor is that in today’s current healthcare setting, at-home tests designed to detect and identify potential MCI are unlikely to be reimbursed by individual and employer health insurance plans if they are neither approved by regulatory agencies, robustly validated, nor directly associated with informing clinical diagnosis and treatment decisions. Given that reality, if a test intended for use at home is associated with a financial cost to either purchase the test or to take the test, widespread use is unlikely. Additionally, insufficient engagement with community memory screening resources (e.g., at senior centers or pharmacies) can limit access to early detection outside of the primary care physician’s (PCP) office.
Barriers Related to Emerging Technological Approaches
Several technological barriers contribute to the challenge of integrating at-home testing into the MCI screening and identification paradigm. Most direct-to-consumer at-home testing options will require a minimum baseline of technological fluency that many older adults may struggle with, which may limit both the use and accuracy of at-home testing. Even patients with the ability to use technology may prefer face-to-face testing with a healthcare provider due to the sensitive nature and potential implications of cognitive testing. While this challenge is likely to decrease in relevance for future generations given the ubiquity of consumer electronics, many older adults today have little familiarity with technology (for example, only using a computer for email, or not at all) and may struggle with an at-home digital test. This challenge may be compounded in patients with comorbid behavioral and psychological symptoms (e.g., apathy), which have been associated with more rapid cognitive decline, higher caregiver burden, and a higher risk of conversion to dementia (4–7). An additional consideration for at-home testing is data privacy concerns both in terms of users being concerned about family members or others seeing them taking a cognitive performance assessment, or the concern that their test results may not stay private. Data security will also become a more daunting issue for at-home tests as more digital technologies gain regulatory approval in the future and are therefore subject to more regulation. Finally, self-administered digital tools introduce variability in testing conditions: in addition to variation in testing environment (e.g., level of background noise, environmental distractions), variation associated with the digital device itself (e.g., type of computer or tablet, operating system and version, recency of software updates) creates uncertainty when considering the accuracy of a cognitive performance assessment.
Parameters of an Ideal Tool
After identifying the most critical barriers to direct-to-consumer assessment of cognitive performance, the working group aligned on potential features of an “ideal” tool to help guide creation or refinement of novel tools.
Test methodology
While multiple direct-to-consumer cognitive evaluations are available, only a minority of assessments explore functional abilities or symptoms associated with cognitive decline (e.g., behavioral symptoms, sleep disorders). The potential for evaluation of functional decline remotely through direct observation by clinicians is certainly possible. As noted in the second publication of this series, an ideal cognitive performance assessment would evaluate across all of these categories. We propose that current cognitive tests would benefit from inclusion of a functional measure and/or questions about cognitive change over time, directed towards a patient and/or an informant. Notably, this robust test methodology is not intended to create an at-home cognitive performance assessment to replace evaluation in a clinical setting. Instead, a well-designed at-home assessment will empower individuals to begin discussing cognitive performance with their PCPs and may help address anxiety about potential cognitive decline among adults with high cognitive performance.
Logistics
For logistics of at-home testing, flexibility is highly favorable, given the variable aptitude and digital fluency of the current generation of older adults. First, the option of having a family member administer a digital or pen-and-paper questionnaire or test to an individual who may not be familiar with consumer electronics may be ideal to maximize the reach and accessibility of at-home testing. We identified relatively few currently-available tests that allow this option; however, the COGSelfTest is designed such that a family member or caregiver can input the user’s answers without impacting the assessment (8, 9). Second, early detection tools should ideally be self-administered or able to be administered by an informant on a range of digital devices, with results either directly forwarded electronically to the PCP office or easily shown to the physician at the next regular appointment. Third, the assessment should be brief, ideally less than 10 minutes, to minimize the perception of the test as a chore or disruptive to daily activities and to reduce attrition of individuals abandoning the test before completion. Many tools meet this criteria, including the BrainCheck assessment, which incorporates multiple established cognitive tests (e.g., Stroop interference test, immediate and delayed recall tasks) into a single 5 – 10 minute session (10).
Score Reporting
Test results should provide the user with information on next steps and available resources if a cognitive impairment is detected. Additionally, an ideal test and score report should allow the user’s test results to be tracked over time – for example, a score report may include a comparison to previous results, when available. Ideally, an at-home resources will be integrated with primary care testing and evaluation. For example, an ideal tool may provide the option for a patient’s score to be sent to their PCP to promote a discussion about cognitive performance at their next routine health visit and to increase the ease with which patients can manage their cognitive performance. Importantly, regardless of performance on the test, the output should include a directive to patients to discuss any cognitive concerns with a PCP.
Validation
Validation of tests and tools in large, diverse populations reflective of the demographics of a real-world population would be ideal. Additionally, at-home cognitive performance assessments should seek to replicate real-world testing environments (i.e., asking users to complete the test at home to replicate distractions, variation in technical ability, and variation in equipment) to understand the accuracy of each assessment in realistic circumstances. At minimum, validation studies should be designed to meet regulatory requirements for marketing authorization.
Optimal care pathway
Integrating direct-to-consumer and/or at-home tests within a clinical care pathway that includes primary care providers is an important step toward establishing the clinical utility of these assessments. Given the nascent stage of development of at-home testing and the range of potential options for its integration with the healthcare system, a multiplicity of viable options to ensure appropriate crosstalk between patients and providers may exist. Importantly, establishing standardized care and referral pathways will be vital, even in the absence of disease-modifying pharmacotherapies, to motivate users, informants, and physicians alike to initiate testing in a manner that allows for appropriate, effective care while also remaining sensitive to the emotional and social impact of MCI and AD. Initiation of at-home testing might occur when an individual or their family members or caregivers have an ongoing concern about cognitive performance and reaches out to their physician. At that point, the physician’s office might provide a recommendation to conduct an at-home test as a first step to see if the concern is warranted without the potentially alarming suggestion to seek an immediate medical opinion. Importantly, the healthcare provider may remind the patient to undergo cognitive testing on a regular basis at home so that a decline can be noted early and can be appropriately captured and communicated to the healthcare provider. As smart home technology offerings expand, they may provide an avenue for monitoring that can be communicated to physicians. Automatic transmission of user results to a healthcare provider would be the optimal strategy to integrate at-home testing with the primary care office. Additionally, artificial intelligence-based technologies may immediately provide scores and clinical labels without requiring transfer of data from the home device to a general server (11). However, privacy concerns associated with these approaches may decrease patient willingness to undergo testing, in addition to the significant infrastructural challenges associated with electronic medical records that remains a persistent challenge for healthcare systems globally. While the pace of development suggests that these challenges can be addressed with time, we encourage test creators to balance potential integration into a clinical care pathway and patient privacy when developing direct-to-consumer or home-based cognitive evaluations.
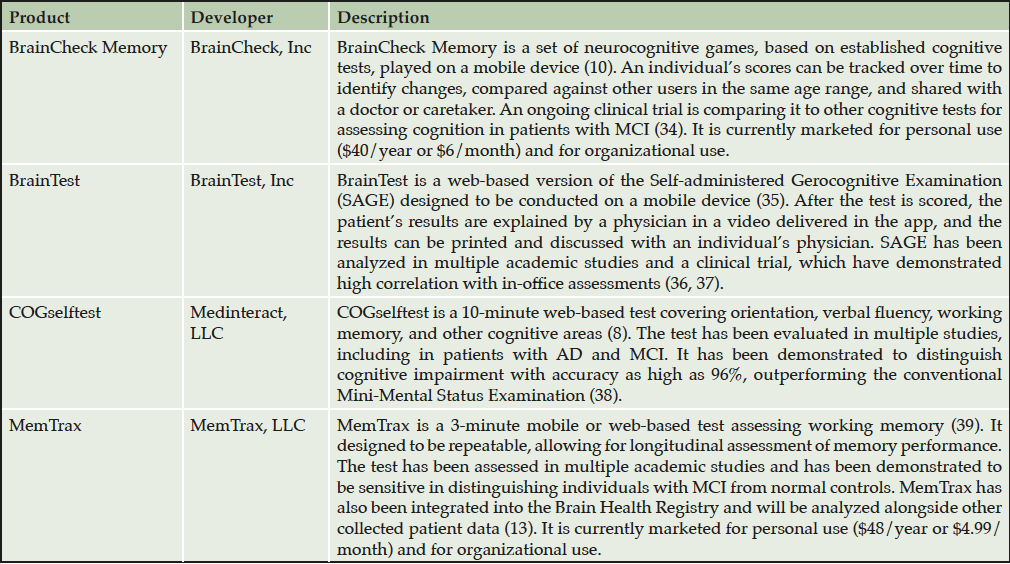
Recent trends and potential disruptors
In recent years, interest in methods to evaluate cognitive performance outside a clinical setting has steadily increased, with countless cognitive performance assessments in development across academia and industry. At-home cognitive assessments, including phone-based and online batteries, have shown promise in identifying individuals who are most likely to have MCI identified through outpatient neuropsychometric testing (12). A variety of assessments are currently available to consumers for at-home use, though levels of clinical validation and interactivity vary (Table 1). Importantly, patient registries like the Brain Health Registry have begun incorporating at-home cognitive tests (13–16), demonstrating that validation of at-home cognitive assessments is an area of ongoing research. Furthermore, a comprehensive set of additional modalities are under active investigation, suggesting that at-home evaluation may ultimately expand from traditional cognitive testing to novel methodologies (Figure 1). Two categories of novel methodologies may be considered: passive technologies that monitor user behavior without active user input and interactive assessments.
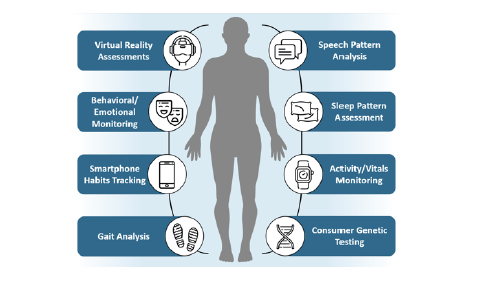
Figure 1. Multiple technologies are under active investigation as methods to detect mild cognitive impairment (MCI) and ultimately improve access to care. Smart devices (e.g., smartphone, fitness tracker, smartwatch, smart-home devices) provide an ideal platform for longitudinal passive data capture on users’ habits and patterns. These data sets may then be analyzed by mobile or online applications to detect subtle changes that may be indicative of decline in cognitive performance. In parallel, active assessments (e.g., virtual reality tools, consumer genetic testing) may help empower users to understand and monitor their own cognitive performance
Passive technologies are of particular interest to the working group given that they could ultimately provide a low-effort, easy-to-use solution for widespread cognitive performance monitoring. Recent academic studies have suggested that subtle changes in behavior, motor function, and cognitive ability are often predictive of future MCI or MCI due to AD (17–20). Indeed, actigraphy-based measurements of behavioral symptoms, including apathy and sleep disorders, may precede cognitive decline (6, 7, 21, 22). Passive monitoring of daily activity via smartwatches, fitness trackers, and smart-home devices could provide a means of tracking behavioral changes over time to detect cognitive decline (23, 24). Select examples of such technologies include the Mindstrong Health application, NeuraMetrix software, and the TATC algorithm. Mindstrong Health has developed a smartphone application that monitors smartphone use to detect behavioral changes associated with mood disorders, with potential to expand to cognitive decline associated with neurodegeneration (25). Similarly, NeuraMetrix has developed software that passively monitors user typing habits to detect changes (e.g., reduced typing speed) associated with cognitive decline (26). The TATC algorithm uses actigraphy to detect behavioral changes associated with abnormal aging and AD (27). These technologies are promising, given the convenient nature of passive monitoring, which does not disrupt a user’s daily routine. Notably, this working group agreed that passive analysis might disrupt the cognitive performance paradigm, resulting in large-scale changes in how we measure cognitive performance, how we monitor patients, and how we understand cognitive performance and aging. In the future, we foresee multiple passive data streams (e.g., smartphone habits, behavioral monitoring, activity monitoring) being integrated into a single application to strengthen early identification of MCI as well as monitor disease progression over time.
In parallel, interactive assessments have undergone extensive investigation in recent years and offer great promise. For example, multiple investigators and companies have created assessments that utilize virtual reality to test a user’s memory, executive functioning, and visuospatial functioning. One notable example is the assessment created by Altoida, in which users navigate a virtual building through a mobile application. Individuals’ performance in this augmented reality environment has been closely correlated with activities of daily living and clinical evaluation of MCI (28, 29). Similarly, many groups have created speech analysis tools that prompt users to perform a verbal task (e.g., describing a photograph) and then utilizes an AI-based algorithm to predict whether users are suffering from cognitive decline. The speech analysis software developed by Winterlight Labs is an example of this approach, which has demonstrated compelling accuracy in detecting MCI and AD in small-scale studies (30, 31). While we perceive these options as less disruptive than passive analysis, we acknowledge that these technologies represent a meaningful step forward with the potential to meaningfully impact patient care.
While we have primarily focused on at-home assessments, we also acknowledge that significant shifts around evaluation of cognitive performance in primary care could dramatically shift the interest in and need for at-home assessments. Blood-based biomarkers of AD are undergoing rapid development and may ultimately be disruptive if an efficient, scalable, and cost-effective technology can be identified and incorporated into primary care. Indeed, there is growing optimism regarding the potential for blood-based biomarkers to detect distinctive AD pathophysiological mechanisms, supported by increasing evidence that core biomarkers and proteins associated with inflammatory and neurodegenerative pathways can be detected in blood (32, 33). Blood-based biomarkers are minimally invasive and time- and resource-effective, thus allowing a decentralized and globally accessible in-vivo biological investigation of AD. While cognitive and functional testing is likely to remain critical even if blood-based biomarkers become widely available, we expect that blood-based biomarker panels will play an increasing central role in future diagnosis and management of AD where disease-modifying therapies agnostic of the clinical stage may be started on a purely biological basis.
Summary
In light of future disease modifying treatments the early detection of MCI in an At-home setting will be mandatory. Significant potential and promising early data exists for at-home detection of MCI. However, the development of digital tools for cognitive evaluation is ultimately still in its infancy. Many tests available today have not yet been validated in large, controlled study settings, thereby preventing widespread adoption and use by concerned adults and physicians. Appropriate regulation of these tools will require updated input from regulatory bodies in this ever-shifting era of digital health. Additionally, the field must carefully consider ethical implications of any algorithm that assesses a user’s personal data, and test creators must strive to respect the privacy and autonomy of any individual user. Test creators must also seek to demonstrate the clinical utility of any at-home test to potentially skeptical physicians and healthcare systems, as integration with current healthcare infrastructure is a critical step toward achieving broad population-level screening and detection, particularly in light of future disease-modifying therapies. Importantly, enhancing holistic patient care and management, irrespective of the availability of a disease-modifying pharmacotherapy, will be equally as important as improving accuracy and timing of the detection of MCI to equip PCPs, patients, and their family members and caregivers with appropriate resources and guidance to cope with MCI.
Despite select barriers that must be addressed, electronic point-of-contact testing holds great promise and will be a critical method to support large-scale cognitive screening for the early detection of MCI, particularly MCI due to AD. Supplementing in-clinic evaluation with at-home assessment may help identify individuals with MCI, allowing physicians to intervene and ultimately to monitor progression, potentially without requiring the individual to present to the physician’s office. When combined with potential improvements to testing in the primary care setting outlined in the second publication of this series, significant potential remains for improving large-scale cognitive screening for the timely and accurate identification of MCI due to AD in a responsible and scalable manner that can be absorbed by healthcare systems.
Acknowledgements and funding: Medical writing support, under the direction of the authors, was provided by ClearView Healthcare Partners, LLC, funded by Eisai Inc., in accordance with Good Publication Practice (GPP3) guidelines.
Disclosures: MNS Royalty: HarperCollins, Stock: uMethod Health, Brain Health, Inc, Optimal Cognitive Health Company, M3 Biosciences, Versanum, NeuroReserveAlzheon and Athira, Speakers Bureau: Peerview and Medscape and HWP, Consultant: Biogen, Bracket/Signant, Neurotrope, Cortexyme, Roche, Grifols, Sanofi, Regeneron, Eisai, Neuronix, Acadia. MB is affiliated with the Research Center and Memory Clinic, Fundació ACE, Institut Català de Neurociències Aplicades, Universitat Internacional de Catalunya, Barcelona, Spain; and with the Networking Research Center on Neurodegenerative Diseases (CIBERNED), Instituto de Salud Carlos III, Spain. Private research funding sources include Grifols SA; Caixabank S.A.; Life Molecular Imaging; Araclon Biotech; Laboratorios Echevarne; Festival Castell Paralada; Bonpreu/Esclat; and Famila Carbó. Public grants include those from Instituto de Salud Carlos III. Ministerio de Salud. Gobierno de España; Dirección General de Farmacia. Ministerio de Salud. Gobierno de España; and European Commission:H2020 program, Innovative Medicine Initiative (IMI-2); and ERA-NET NEURON program, European Marie Sklodowska Curie. Advisory work includes that for Araclon Biotech, Biogen, Bioibérica, Eisai, Grifols, Lilly, Merck, Nutricia, Roche, Oryzon, Schwabe Farma, Servier, and Kyowa Kirin. PMD has received research grants (through Duke University) from Avid, Lilly, Neuronetrix, Avanir, Bauch, Alzheimer’s Drug Discovery Foundation, Cure Alzheimer’s Fund, Wrenn Trust, DOD, ONR, and NIH. PMD has received speaking or advisory fees from Anthrotronix, Neuroptix, Genomind, Clearview, Cognicity, Nutricia, Living Media, Verily, RBC, Brain Canada, and CEOs Against Alzheimers. PMD owns shares in Muses Labs, Anthrotronix, Evidation Health, Turtle Shell Technologies and Advera Health Analytics whose products are not discussed here. He has received travel support from World Economic Forum, CCABH and Canaan Ventures. PMD served/serves on the board of Baycrest, AHEL, TLLF and TGHF. PMD is a co-inventor (through Duke) on patents relating to dementia biomarkers and therapies. BD is affiliated with the Institute of Memory and Alzheimer’s Disease (IM2A), Department of Neurology, Salpêtrière Hospital, AP-HP, Sorbonne-Université, Paris, France. JI is affiliated as an Assistant Professor-Adjunct (Group1) with the Department of Family Practice at Queen’s University. She was also a panelist for Hoffman-La Roche Limited, Ottawa, on an Alzheimer’s Disease Panel. She is involved with the Canadian Consortium on Neurodegeneration in Aging (CCNA), Extension Study as a Co- Investigator and Research Coordinator. AI received lecture fees from Eisai, Janssen, Otsuka, Eli Lilly, MSD, Chugai-Roche, Daiichi-Sankyo, Alnylam, Takeda, UCB, Ono, Integra Japan, IQVIA, Fuji Rebio, Biogen, and advisory fees from Janssen during the past three years. AP reports personal fees from Acadia Pharmaceuticals, Functional Neuromodulation, Neurim Pharmaceuticals, Grifols, Eisai, BioXcel, Tetra Discovery Partners, and Merck; grants from AstraZeneca, Avanir, Biogen, Biohaven, Eisai, Eli Lilly, Janssen, Genentech/Roche, Novartis, Merck, as well as funding from NIA, NIMH, DOD. KLP receives grant funding from the National Institute on Aging, the National Institute of Neurological Disorders and Stroke, the Global Brain Health Institute, and Quest Diagnostics. BV has consultancy and research grants from Roche, Biogen, EISAI, Nestle, Lilly, Cerecin, and Merck. HH is an employee of Eisai Inc. and serves as Senior Associate Editor for the Journal Alzheimer’s & Dementia; during the past three years he had received lecture fees from Servier, Biogen and Roche, research grants from Pfizer, Avid, and MSD Avenir (paid to the institution), travel funding from Eisai, Functional Neuromodulation, Axovant, Eli Lilly and company, Takeda and Zinfandel, GE-Healthcare and Oryzon Genomics, consultancy fees from Qynapse, Jung Diagnostics, Cytox Ltd., Axovant, Anavex, Takeda and Zinfandel, GE Healthcare, Oryzon Genomics, and Functional Neuromodulation, and participated in scientific advisory boards of Functional Neuromodulation, Axovant, Eisai, Eli Lilly and company, Cytox Ltd., GE Healthcare, Takeda and Zinfandel, Oryzon Genomics and Roche Diagnostics. He is co-inventor in the following patents as a scientific expert and has received no royalties: • In Vitro Multiparameter Determination Method for the Diagnosis and Early Diagnosis of Neurodegenerative Disorders Patent Number: 8916388; • In Vitro Procedure for Diagnosis and Early Diagnosis of Neurodegenerative Diseases Patent Number: 8298784; • Neurodegenerative Markers for Psychiatric Conditions Publication Number: 20120196300; • In Vitro Multiparameter Determination Method for The Diagnosis and Early Diagnosis of Neurodegenerative Disorders Publication Number: 20100062463; • In Vitro Method for The Diagnosis and Early Diagnosis of Neurodegenerative Disorders Publication Number: 20100035286; • In Vitro Procedure for Diagnosis and Early Diagnosis of Neurodegenerative Diseases Publication Number: 20090263822; • In Vitro Method for the Diagnosis of Neurodegenerative Diseases Patent Number: 7547553; • CSF Diagnostic in Vitro Method for Diagnosis of Dementias and Neuroinflammatory Diseases Publication Number: 20080206797; • In Vitro Method for The Diagnosis of Neurodegenerative Diseases Publication Number: 20080199966; • Neurodegenerative Markers for Psychiatric Conditions Publication Number: 20080131921. AV is an employee of Eisai Inc. and received lecture honoraria from Roche, MagQu LLC, and Servier.
Open Access: This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, duplication, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
References
1. Petersen RC, Caracciolo B, Brayne C, Gauthier S, Jelic V, Fratiglioni L. Mild cognitive impairment: a concept in evolution. J Intern Med 2014;275:214–228
2. Jack CR, Bennett DA, Blennow K, et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement J Alzheimers Assoc 2018;14:535–562
3. Medical Care Corporation: MCI Screen. http://www.mciscreen.com/. Accessed 1 Nov 2019
4. Lyketsos CG, Carrillo MC, Ryan JM, et al. Neuropsychiatric symptoms in Alzheimer’s disease. Alzheimers Dement J Alzheimers Assoc 2011;7:532–539
5. Lyketsos CG, Lopez O, Jones B, Fitzpatrick AL, Breitner J, DeKosky S. Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: results from the cardiovascular health study. JAMA 2002;288:1475–1483
6. Kuhlmei A, Walther B, Becker T, Müller U, Nikolaus T. Actigraphic daytime activity is reduced in patients with cognitive impairment and apathy. Eur Psychiatry J Assoc Eur Psychiatr 2013;28:94–97
7. David R, Mulin E, Friedman L, et al. Decreased daytime motor activity associated with apathy in Alzheimer disease: an actigraphic study. Am J Geriatr Psychiatry Off J Am Assoc Geriatr Psychiatry 2012;20:806–814
8. COGselftest → Alzheimer’s and Dementia Cognitive Screening Test. http://cogselftest.com/. Accessed 14 Aug 2019
9. de Leonni Stanonik M, Licata CA, Walton NC, Lounsbury JW, Hutson RK, Dougherty JH. The Self Test: a screening tool for dementia requiring minimal supervision. Int Psychogeriatr 2005;17:669–678
10. BrainCheck | Mobile Neurocognitive Testing Solutions. BrainCheck. https://braincheck.com/. Accessed 14 Aug 2019
11. Piau A, Wild K, Mattek N, Kaye J. Current State of Digital Biomarker Technologies for Real-Life, Home-Based Monitoring of Cognitive Function for Mild Cognitive Impairment to Mild Alzheimer Disease and Implications for Clinical Care: Systematic Review. J Med Internet Res 2019;21:e12785
12. Sano M, Zhu CW, Kaye J, et al. A randomized clinical trial to evaluate home-based assessment of people over 75 years old. Alzheimers Dement J Alzheimers Assoc 2019;15:615–624
13. Weiner MW, Nosheny R, Camacho M, et al. The Brain Health Registry: An internet-based platform for recruitment, assessment, and longitudinal monitoring of participants for neuroscience studies. Alzheimers Dement J Alzheimers Assoc 2018;14:1063–1076
14. Home | Brain Health Registry. https://www.brainhealthregistry.org/. Accessed 15 Oct 2019
15. Nosheny RL, Camacho MR, Insel PS, et al. Online study partner-reported cognitive decline in the Brain Health Registry. Alzheimers Dement N Y N 2018;4:565–574
16. Mackin RS, Insel PS, Truran D, et al. Unsupervised online neuropsychological test performance for individuals with mild cognitive impairment and dementia: Results from the Brain Health Registry. Alzheimers Dement Amst Neth 2018;10:573–582
17. Fernández G, Manes F, Politi LE, et al. Patients with Mild Alzheimer’s Disease Fail When Using Their Working Memory: Evidence from the Eye Tracking Technique. J Alzheimers Dis JAD 2016;50:827–838
18. Zola SM, Manzanares CM, Clopton P, Lah JJ, Levey AI. A behavioral task predicts conversion to mild cognitive impairment and Alzheimer’s disease. Am J Alzheimers Dis Other Demen 2013;28:179–184
19. Naismith SL, Mowszowski L. Sleep disturbance in mild cognitive impairment: a systematic review of recent findings. Curr Opin Psychiatry 2018;31:153–159
20. Bahureksa L, Najafi B, Saleh A, et al. The Impact of Mild Cognitive Impairment on Gait and Balance: a Systematic Review and Meta-Analysis of Studies using Instrumented Assessment. Gerontology 2017;63:67–83
21. Mulin E, Zeitzer JM, Friedman L, et al. Relationship between apathy and sleep disturbance in mild and moderate Alzheimer’s disease: an actigraphic study. J Alzheimers Dis JAD 2011;25:85–91
22. Zeitzer JM, David R, Friedman L, et al. Phenotyping apathy in individuals with Alzheimer disease using functional principal component analysis. Am J Geriatr Psychiatry Off J Am Assoc Geriatr Psychiatry 2013;21:391–397
23. Dodge HH, Zhu J, Mattek NC, Austin D, Kornfeld J, Kaye JA. Use of High-Frequency In-Home Monitoring Data May Reduce Sample Sizes Needed in Clinical Trials. PLoS ONE 2015;10
24. Jekel K, Damian M, Storf H, Hausner L, Frölich L. Development of a Proxy-Free Objective Assessment Tool of Instrumental Activities of Daily Living in Mild Cognitive Impairment Using Smart Home Technologies. J Alzheimers Dis JAD 2016;52:509–517
25. Home. Mindstrong Health. https://mindstronghealth.com/. Accessed 14 Oct 2019
26. NeuraMetrix – Measuring Brain Health. NeuraMetrix. https://www.neurametrix.com. Accessed 14 Oct 2019
27. Li J, Yu R, Meng H, Lu Z, Kwok T, Cheng H. TATC: Predicting Alzheimer’s Disease with Actigraphy Data. KDD ’18 24th ACM SIGKDD Int Conf Knowl Discov Data Min 2018
28. Altoida – Know Thyself – We assess your Brain Health!
29. Tarnanas I, Tsolaki A, Wiederhold M, Wiederhold B, Tsolaki M. Five-year biomarker progression variability for Alzheimer’s disease dementia prediction: Can a complex instrumental activities of daily living marker fill in the gaps? Alzheimers Dement Amst Neth 2015;1:521–532
30. Winterlight Labs. https://winterlightlabs.com/. Accessed 14 Oct 2019
31. Fraser KC, Meltzer JA, Rudzicz F. Linguistic Features Identify Alzheimer’s Disease in Narrative Speech. J Alzheimers Dis JAD 2016;49:407–422
32. Hampel H, O’Bryant SE, Molinuevo JL, et al. Blood-based biomarkers for Alzheimer disease: mapping the road to the clinic. Nat Rev Neurol 2018;14:639–652
33. Henriksen K, O’Bryant SE, Hampel H, et al. The future of blood-based biomarkers for Alzheimer’s disease. Alzheimers Dement J Alzheimers Assoc 2014;10:115–131
34. Video Games to Track Cognitive Health – Full Text View – ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT03608722. Accessed 14 Oct 2019
35. BrainTest® – Online Dementia, MCI & Alzheimer’s Screening Test. BrainTest. https://braintest.com/. Accessed 14 Oct 2019
36. Validity of the Electronic Self-Administered Gerocognitive Examination (eSAGE) – Full Text View – ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT02544074. Accessed 14 Oct 2019
37. Scharre DW, Chang SI, Nagaraja HN, Vrettos NE, Bornstein RA. Digitally translated Self-Administered Gerocognitive Examination (eSAGE): relationship with its validated paper version, neuropsychological evaluations, and clinical assessments. Alzheimers Res Ther 2017;9:44
38. Dougherty JH, Cannon RL, Nicholas CR, et al. The computerized self test (CST): an interactive, internet accessible cognitive screening test for dementia. J Alzheimers Dis JAD 2010;20:185–195
39. The Online Memory Test. MemTrax. https://memtrax.com/. Accessed 14 Oct 2019