S. Abushakra1, A. Porsteinsson2, B. Vellas3, J. Cummings4, S. Gauthier5, J.A. Hey1, A. Power1, S. Hendrix6, P. Wang7, L. Shen7, J. Sampalis8, M. Tolar1
1. Alzheon Inc., Framingham, MA, USA; 2. University of Rochester, Rochester, NY, USA; 3. University of Toulouse, Toulouse, France; 4. Cleveland Clinic Lou Ruvo Center for Brain Health, Las Vegas, NV, USA; 5. McGill University and McGill Center for Studies in Aging, Montreal, Canada; 6. Pentara Corporation, Salt Lake City, UT, USA; 7. Pharmapace Inc., San Diego, CA, USA; 8. McGill University and JSS Medical Research Inc., Montreal, Canada
Corresponding Author: Susan Abushakra, MD, Alzheon, Inc., 111 Speen Street, Suite 306, Framingham, MA 01701, USA, Phone: 508.508.7709, Fax: 508.861.1500, susan.abushakra@alzheon.com
J Prev Alz Dis 2016;3(4):219-228
Published online October 24, 2016, http://dx.doi.org/10.14283/jpad.2016.115
Abstract
Background: Tramiprosate is an oral amyloid anti-aggregation agent that reduces amyloid oligomer toxicity in preclinical studies and was evaluated in two 78-week trials in North America and Western Europe that enrolled 2,025 patients with Mild to Moderate Alzheimer’s Disease. The completed North American study did not achieve its efficacy objectives, but a pre-specified subgroup analysis suggested potential efficacy in apolipoprotein E4 (APOE4) carriers. To further explore this observation, we analyzed tramiprosate Phase 3 clinical data based on the number of APOE4 alleles.
Objectives: To analyze tramiprosate efficacy, safety, and occurrence of vasogenic edema in the three APOE4 subgroups: homozygous, heterozygous and non-carriers.
Design: Randomized, double-blind, placebo-controlled parallel-arm multi-center studies.
Setting: Academic Alzheimer’s disease & dementia centers, community-based dementia and memory clinics, and neuropsychiatric clinical research sites.
Participants: Subjects included 2,025 patients, 50 years of age or older, with approximately 60% having APOE4 carrier status (10-15% homozygotes and 45-50% heterozygotes), and mild to moderate disease. All subjects were on stable symptomatic drugs.
Intervention: Randomized subjects received placebo, 100 mg BID, or 150 mg BID of tramiprosate.
Measurements: Co-primary outcomes in both studies were change from baseline in the ADAS-cog11 and CDR-SB assessment scales.
Results: Highest efficacy was observed in APOE4/4 homozygotes receiving 150 mg BID of tramiprosate, showing statistically significant effects on ADAS-cog and positive trends on CDR-SB (respectively, 40-66% and 25-45% benefit compared to placebo). APOE4 heterozygotes showed intermediate efficacy, and non-carriers showed no benefit. In 426 patients with MRI scans, no cases of treatment-emergent vasogenic edema were observed. In the three subgroups, the most common adverse events were nausea, vomiting, and decreased weight.
Conclusions: The “APOE4 Gene-Dose effect” is likely explained by the high prevalence of amyloid pathology in symptomatic APOE4 carriers. In APOE4/4 Alzheimer’s disease patients, the high dose of tramiprosate showed favorable safety and clinically meaningful efficacy in addition to standard of care.
Key words: Tramiprosate, Alzheimer’s, APOE4.
Abbreviations: AD: Alzheimer’s disease; Aβ: Beta amyloid; ADAS-cog: Alzheimer’s Disease Assessment Scale-cognitive subscale; APOE4: Apolipoprotein E4, ε4 allele of the apolipoprotein E gene; ARIA-E: Amyloid-Related Imaging Abnormalities-Edema; ARIA-H: Amyloid-Related Imaging Abnormalities-Haemosiderin; CBL: Change from Baseline; CDR-SB: Clinical Dementia Rating Scale-Sum of Boxes; DAD: Disability Assessment for Dementia; EU: European; FLAIR: Fluid-Attenuated Inversion Recovery; ITT: Intent to Treat; MedDRA: Medical Dictionary for Regulatory Activities; MMRM: Mixed Effects Repeated Measures Model; MMSE: Mini-Mental State Examination; MRI: Magnetic Resonance Imaging; NA: North American; NPI: Neuropsychiatric Inventory; OC: Observed Cases; SAE: Serious Adverse Event; SAP: Statistical Analysis Plan; TEAE: Treatment-Emergent Adverse Events
Introduction
The ε4 allele of the apolipoprotein E gene (APOE4) is the most important genetic risk factor for Alzheimer’s disease (AD), second only to age in determining the risk for developing AD dementia (1). APOE alleles encode carrier proteins that are important in cholesterol and β-amyloid (Aβ) metabolism and clearance, with the APOE4 isoform showing reduced clearance of Aβ peptides and promoting their aggregation (2). APOE4 carriers have deficient Aβ clearance from brain and excessive amyloid deposition associated with markers of neurodegeneration (3). The APOE4 genotype confers a 4-fold to 12-fold higher risk of developing AD, and decreases the mean age of onset of AD by approximately 10-15 years (4, 5). APOE4 carriers also show more rapid progression from early AD to dementia (6). Neuroimaging as well as neuropathological and cerebrospinal fluid (CSF) samples from AD patients who are APOE4 carriers indicate high levels of Aβ pathology with amyloid deposition in both cortex and cerebral vasculature (3, 7, 8). The high burden of vascular amyloid in APOE4 carriers has been associated with increased risk of vasogenic edema and microhemorrhage or Amyloid Related Imaging Abnormalities (ARIA-E or ARIA-H) with some amyloid-targeted agents (9). Due to the susceptibility of APOE4 carriers, especially homozygotes, to occurrence of ARIA, clinical trials with most amyloid targeted agents now include APOE4 genotyping and brain MRI as a safety evaluation. Amyloid PET-imaging studies of patients with mild cognitive changes have shown highest rates of positive scans in homozygotes, intermediate rates in heterozygotes, and lowest rates in non-carriers (10). Similar findings were reported in Mild to Moderate AD patients (11). It is therefore plausible that an amyloid-targeted AD drug could have differential efficacy based on the number of APOE4 alleles in treated subjects. We evaluated clinical data from two Phase 3 trials with tramiprosate based on the number of APOE4 alleles.
Tramiprosate is an oral amyloid anti-aggregation agent that reduces oligmeric and fibrillar (plaque) amyloid in transgenic animal models (12, 13). In a Phase 2 study in AD patients, tramiprosate was found to cross the blood-brain barrier and to dose-dependently reduce CSF Aβ42 levels, with maximum reductions at the highest tested dose of 150 mg BID (14). This allowed selection of 100 mg BID and 150 mg BID doses for the Phase 3 program. The Phase 3 program included two similarly designed, 78-week trials in patients with mild to moderate AD. The North American (NA) trial results that became available in 2007 did not show significant efficacy on the Alzheimer’s Disease Assessment Scale-cognitive subscale (ADAS-cog) and Clinical Dementia Rating Scale-Sum of Boxes (CDR-SB) co-primary outcomes (15). The European (EU) trial was therefore terminated prior to completion and the study results were not previously published. In both trials the diagnosis of AD was made based on clinical criteria, with brain MRI or CT imaging as supportive evidence. APOE4 status was one of the pre-specified covariates which was found to be significant for both primary outcomes (p < 0.05), and an initial analysis showed an efficacy trend in APOE4 carriers. We have further systematically analyzed the primary, secondary and safety outcomes of both studies based on the number of APOE4 alleles in APOE4 homozygous (2 alleles), heterozygous (1 allele) and non-carrier patients (0 alleles).
Methods
Study Design
The NA study was a randomized, placebo-controlled, double-blind, parallel-arm study (Study CL-758007) which enrolled subjects with baseline Mini-Mental State Examination (MMSE) of 16-26, inclusive, at 67 centers in the US and Canada. Subjects were randomized into one of three arms (placebo, 100 mg BID and 150 mg BID) and received study drug for 78 weeks, with visits occurring every 13 weeks (15). Subjects were allowed stable doses of cholinesterase inhibitors, alone or with memantine, for at least 12 weeks. The EU study (CL-758010) had a similar design, but prohibited memantine use, and enrolled subjects from 10 European countries. Co-primary outcomes of both studies were changes from baseline (CBL) to week 78 on the 11-item ADAS-cog (16) and on the CDR-SB (17). The CDR-SB combines cognitive and functional measures. Secondary outcomes included the Disability Assessment for Dementia (DAD) (18), the MMSE (19), and the 12-item Neuropsychiatric Inventory (NPI) (20) which evaluates neuropsychiatric symptoms that are common in dementia patients.
Clinical Data Analyses
APOE4 status was one of the pre-specified covariates which was found to be significant for both primary outcomes (p < 0.05). In addition, there was an overall significant treatment by APOE4 by visit interaction for ADAS-cog (p < 0.05). Treatment by APOE4 interaction at individual visits was significant for both outcomes at weeks 52 and 65, and for CDR-SB at week 78 as well (p < 0.05). These results suggest that placebo-corrected treatment effects are dependent on APOE4 status. Therefore an analysis in APOE4 carriers and non-carriers was performed, and showed an efficacy trend in APOE4 carriers.
Efficacy and safety data from each study were analyzed based on APOE4 genotype status in homozygous patients (APOE4/4 genotype), heterozygous (mostly APOE3/4) and APOE4 non-carriers (mostly APOE3/3). Safety analyses for treatment-emergent adverse events (TEAE) and serious adverse event (SAE) were analyzed in each study and in the combined dataset. Adverse events were coded according to MedDRA version 9.0.
Centralized Brain MRI Scan Analyses
Both studies included brain MRI imaging in a subset of patients for volumetric assessments (21), but centralized MRI assessments for vasogenic edema were not planned since the occurrence of ARIA in AD trials was not yet described. Blinded paired MRI scans were available for ARIA assessment. MRI scans were performed at screening and at week 78 or at the early termination visit, if the patient discontinued after 6 months in study. The scans included Fluid-Attenuated Inversion Recovery (FLAIR) sequences that were used for ARIA-E assessments. Subjects who had MRI scans after study treatment only were included in the analysis since spontaneous ARIA-E is uncommon. Neuroradiologists (Synarc/BioClinica®), who were blinded to patients’ treatment and genotype, evaluated all scans according to a standardized protocol.
Statistical Analyses
Efficacy analyses
Two independent biostatistical consulting groups were retained to independently conduct the efficacy analyses to ensure reproducibility (Pharmapace, Inc., San Diego CA and Pentara Corporation, Salt Lake City UT). Both groups started with the same raw datasets and agreed on conventions for visit windows and pooling of study sites with low subject numbers. The mixed effects repeated measures model (MMRM) was the analytical method specified in the statistical analysis plan (SAP) of the NA study. This SAP was developed by JSS Medical Research Inc., and had undergone Food and Drug Administration review prior to database lock. This MMRM included the following terms: treatment, visit, treatment by visit interaction, study site, and baseline value of the primary endpoint to be analyzed. The analyses were performed using this “original” MMRM. Since it is now known that baseline disease severity may influence drug effect, current AD studies include baseline disease severity in the MMRM model. Therefore, an additional analysis was performed that included baseline MMSE in the model (“updated MMRM”). In addition, due to smaller sample sizes in the APOE4 subgroups, study sites were pooled for sites recruiting few patients to avoid having only one subject at a site in a dose arm from an APOE4 subgroup. Both MMRM methods yielded similar results and conclusions.
The NA study SAP included the following factors as covariates to be tested for interaction with drug effect: age, gender, use of symptomatic drugs, and APOE4 genotype; and required that efficacy be summarized by APOE genotype.
The EU study was prematurely discontinued when the NA study results became available and the program was terminated. Most patients in the EU study had completed week 52 when the NA study results became public, while approximately 30% had completed week 65 and week 78. Therefore, the last two visits had a small sample size, and the evaluations were possibly biased since they were also termination visits after public release of the data (temporal bias). The MMRM method that includes efficacy from all visits may not be appropriate for the EU study, due to the introduction of potentially biased data from the later visits. EU efficacy results are presented using summary descriptive statistics based on observed cases (OC analysis) without imputations for missing data.
Efficacy analyses are shown for subjects in the intent to treat (ITT) population who had a baseline assessment and at least one post-baseline assessment, received at least one dose of study drug, and had a known APOE genotype. The pooled safety population included all subjects who received at least one dose of study drug regardless of APOE genotype status.
In the NA study, sensitivity analyses were performed using the completer set (subjects who completed week 78) and summary statistics using OC analysis. Exploratory analyses were performed that evaluated efficacy on ADAS-cog, CDR-SB, and DAD for 3 MMSE categories (16-26; 20-26; 22-26), using the same MMRM method as the primary analysis. Since these APOE4 subgroup analyses are used for hypothesis generation to inform future studies, the p-values presented were not adjusted for multiple statistical comparisons.
Specification of Model Variables (MMRM)
Response Variables
CBL(ADAS-cog)Visit i = (ADAS-cog)Visit i – (ADAS-cog)Baseline
CBL(CDR-SB)Visit i = (CDR-SB)Visit i – (CDR-SB)Baseline
Independent Variables
The treatment group was regarded as a class variable with three levels: (1) tramiprosate 150 mg BID, (2) tramiprosate 100 mg BID, and (3) placebo. The visit variable was included as a class variable with values standing for the visit numbers (visit 5 to visit 10, corresponding to weeks 13 to 78), and was used to index the within-subject observations over time. Study site (SITE) was entered in the model as a categorical, non-ordinal variable.
Covariates
Baseline value (BASE) of the dependent variable was entered as a continuous covariate in order to account for the fact that both absolute and percent changes over time in the outcome measure correlate with its value at the baseline visit. Baseline MMSE score was entered as a continuous covariate. The autoregressive order 1 (AR1) covariance structure was used to model the within-subject correlation across the different visits.
Results
Demographics and Baseline Characteristics
The NA and EU studies enrolled 1,052 and 973 subjects, respectively. The ITT population for these analyses included 973 and 870 subjects from the NA and EU studies with known APOE genotypes. Patient demographics and baseline values in the APOE4 subgroups (NA Study) are shown in Table 1.
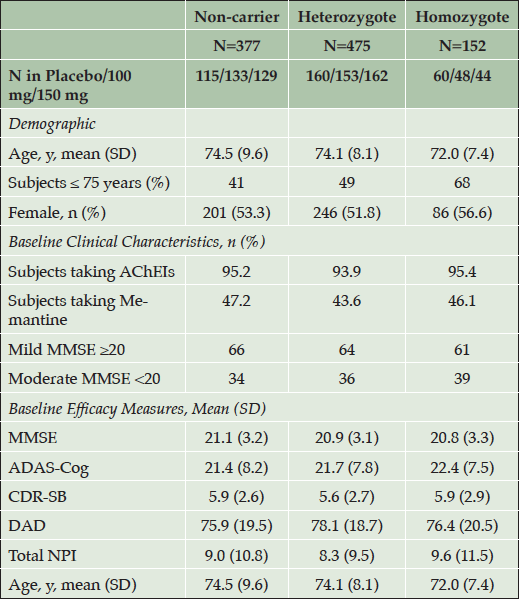
Table 1. Patient Demographic and Baseline Clinical Characteristics in APOE4 Subgroups (NA Study, All Enrolled)
Higher values for ADAS-cog, CDR-SB and NPI indicate greater severity, while lower values in DAD and MMSE indicate greater severity. APOE4 = apolipoprotein E4; AChEIs = acetylcholinesterase inhibitors; MMSE = Mini Mental State Examination; ADAS-Cog = Alzheimer’s Disease Assessment Scale-cognitive subscale; CDR-SB = Clinical Dementia Rating Sum of Boxes; DAD = Disability Assessment for Dementia; NPI = Neuropsychiatric Inventory.
In the NA study, baseline demographics of the three APOE4 groups were balanced except for the APOE4/4 group having a younger mean age and highest proportion of patients under age 75 years. The younger age in APOE4/4 patients is consistent with established epidemiologic observations and attributed to earlier amyloid accumulation and onset of symptoms in this population (10, 22).
The APOE4/4 group also had a higher proportion of patients in the moderate AD category with 39% versus 34% in the non-carriers subgroup. In the APOE4/4 sub-group the mean baseline ADAS-cog score is slightly higher than in non-carriers, while the mean CDR-SB score is similar.
In the APOE4/4 subgroup, which showed an efficacy signal, baseline ADAS-cog and CDR-SB scores in the 3 treatment arms are shown (Table 2). Mean ADAS-cog is higher and CDR-SB is lower in the low dose arm than in the other two arms. The MMRM model adjusts for baseline severity by including the baseline score of the outcome measure as a covariate in the analysis.
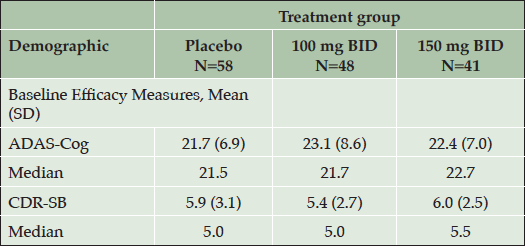
Table 2. Baseline ADAS-Cog and CDR-SB Scores in the APOE4/4 Homozygous Subgroup (NA Study, ITT Population)
Higher values for ADAS-cog and CDR-SB indicate greater severity. ADAS-Cog = Alzheimer’s Disease Assessment Scale-cognitive subscale; CDR-SB = Clinical Dementia Rating Sum of Boxes
Efficacy
Co-Primary Outcomes in the APOE4 Subgroups
The primary analyses of the 11-item ADAS-cog and CDR-SB are shown for each APOE4 subgroup in the ITT population with the least squares mean differences between each dose and placebo shown at week 65 and week 78 (the primary endpoint). The results of the updated MMRM model are shown in Table 3. The p-values presented were not adjusted for multiple statistical comparisons and are thus considered nominal p-values.
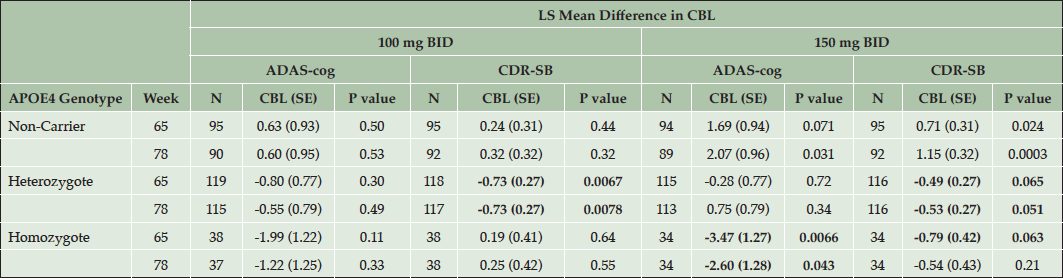
Table 3. Effects of Tramiprosate on ADAS-Cog and CDR-SB in APOE4 Subgroups (NA Study, ITT Population, N=973)
Note: Change from Baseline (CBL), is the LS mean differences in ADAS, CDR-SB between active arm and placebo. Negative values for LS mean differences in CBL of ADAS and CDR-SB indicate clinical benefit in favor of drug. Bolded values indicate clinical benefit with either nominal statistical significance (p < 0.05) or positive trends (p values between 0.05 and 0.1).
In APOE4/4 homozygotes in the high dose arm, a significant drug-placebo difference was observed on ADAS-cog at the last visits. The CDR-SB shows numerical benefit at week 78 and a positive trend at week 65 (p = 0.063). In the homozygous group in the low dose arm, there is numerical benefit on ADAS-cog and non-significant effects on CDR-SB at weeks 65 and 78. The APOE4 heterozygous group shows non-significant drug effects on ADAS-cog at both doses, but shows significant benefit on CDR-SB at the low dose, and a positive trend at the high dose. The non-carrier group shows non-significant effects on both outcomes at the low dose. In the non-carrier group placebo is significantly better than 150 mg tramiprosate, the high dose. The APOE4/4 homozygous group on high dose tramiprosate, therefore, shows the most consistent benefit on co-primary outcomes. Figure 1A and 1B shows the efficacy results in the APOE4 subgroups on the high dose at the last visits.
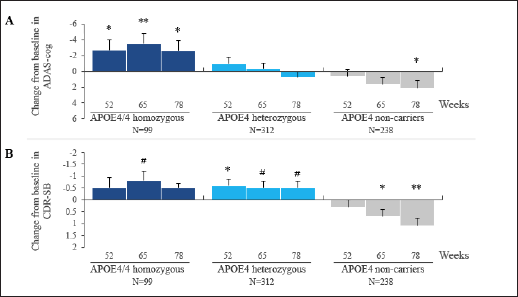
Figure 1. Effects of Tramiprosate 150 mg BID in APOE4 Subgroups on Co-Primary Outcomes, ADAS-cog (Panel A) and CDR-SB (Panel B). Change from Baseline (CBL): LS mean differences between active arm and placebo are shown at weeks 52, 65 and 78 (ITT, MMRM Analysis), error bars are standard error of mean. Negative CBL indicates clinical benefit for both outcomes
P values: *p < 0.05, **p < 0.01 (indicate nominal statistical significance) and #p < 0.1 (indicates positive trend).
Efficacy Analyses in APOE4/4 Homozygous Patients: Time Course of Effect in NA Study
The time course of treatment effects in the APOE4/4 subgroup is shown in Figure 2 (panels A-B). At the high dose, the ADAS-cog shows a progressive separation from placebo starting at week 26 and reaching the largest drug-placebo difference at week 65; this treatment effect remains significant at weeks 52-78. The CDR-SB shows a similar time course, with the drug-placebo difference showing a positive trend at week 26 and again at week 65. At the low dose, the ADAS-cog shows a biphasic effect with early significant benefit at week 26 and numerical benefit at weeks 65 and 78, that is smaller than the high dose effect. The CDR-SB at the low dose shows minimal separation from placebo.
Sensitivity Analyses of Co-Primary Outcomes in APOE4/4 Homozygous Patients (NA Study)
Efficacy analysis in the completers showed similar results to the ITT analysis (data not shown). Descriptive statistics in the OC analysis also showed similar results to the MMRM analysis, as shown in Figure 2 (panels C-D).
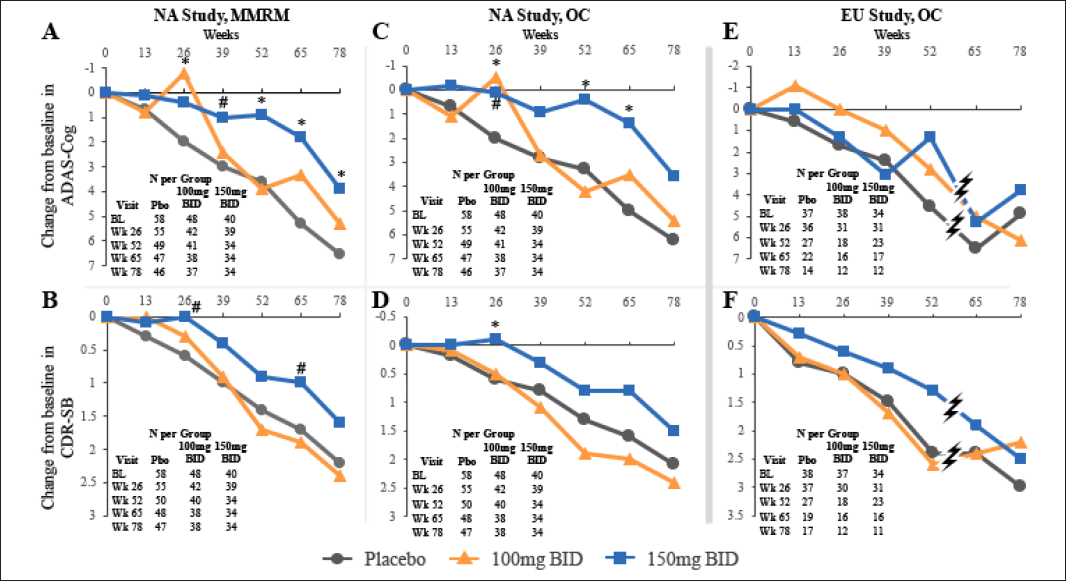
Figure 2. Time Course of Tramiprosate Effects on co-primary outcomes in APOE4/4 Population at 100 mg and 150 mg BID in NA (Panels A-D) and EU studies (Panels E, F). NA study panels A and B show MMRM analysis, C and D show summary statistics with observed cases (OC). EU study panels E and F show summary statistics with observed cases. (ITT population, NA study: N= 147; EU study: N= 110). For both ADAS-cog and CDR-SB, positive CBL (downward graph) indicates worsening.
P values: *p < 0.05 (indicate nominal statistical significance) and # p < 0.1 (indicates positive trend); The hashes in panels E and F, indicate that the study was terminated at the time when most patients had completed week 52 visit, but only 30% had completed week 65 and week 78 visits.
Primary and Secondary Efficacy Outcomes in APOE4 Homozygous Patients and Percent Benefit Compared to Placebo (NA Study)
To further understand the efficacy in the APOE4/4 homozygous subgroup, effects of the high dose on the co-primary and key secondary outcomes (DAD, NPI, MMSE) were analyzed by determining the percent benefit compared to placebo (Table 4). The secondary outcomes were assessed at weeks 25, 52 and 78, and are presented at the last 3 visits, together with ADAS and CDR. The percent benefit compared to placebo is calculated as: [CBL drug – CBL placebo]/CBL placebo. Note that percent benefit is indicated as positive if the result favors drug (i.e. negative LS mean differences for ADAS, CDR, and NPI; and positive LS mean differences for DAD and MMSE).
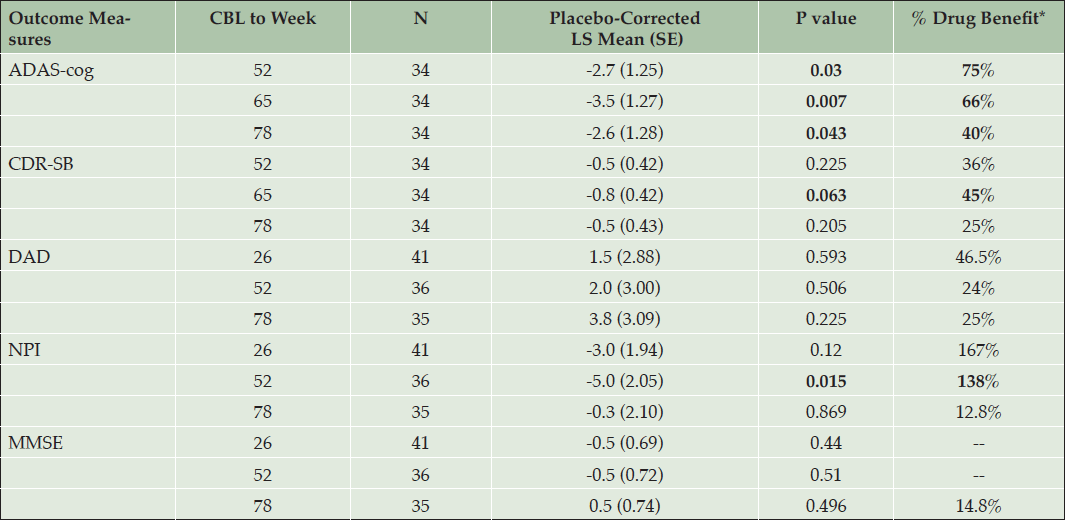
Table 4. Effect of Tramiprosate 150 mg BID in APOE4/4 homozygous group on primary and secondary outcomes and % drug benefit compared to placebo (NA study, ITT)
* % Drug benefit is indicated as positive when drug is better than placebo. For ADAS, CDR, and NPI negative LS means differences favor active drug, for DAD and MMSE positive differences favor active drug. Bolded values indicate clinical benefit with either nominal statistical significance (p < 0.05) or positive trends: p values between 0.05 and 0.1
The DAD showed consistent numerical benefit at weeks 26, 52, and 78. The NPI showed numerical benefit at endpoint, and a positive trend at week 52 (p <0.05). The MMSE showed small effects that were not significant at these same visits.
Efficacy Analysis in APOE4/4 Homozygous Patients: Time Course of Effect in EU Study
The baseline demographics of the three APOE4 groups were balanced except for the APOE4/4 group having a younger mean age, and highest proportion of patients under age 75 years (58% under age 75 years in non-carriers, 64% in heterozygous, and 77% in APOE4/4 groups). The APOE4/4 group also had the highest proportion of patients in the mild AD category (68% mild AD in non-carriers, 64% in heterozygous, and 74% in APOE4/4 groups).
Summary statistics with observed cases are shown for both outcomes in Figure 2 (panels E-F). In the OC analysis the ADAS-cog effect at the low dose shows numerical benefit at all visits up to week 52. At the high dose results suggest benefit over placebo at week 52, but not at earlier time points that showed positive trends in the NA study. The effect at week 52 is similar in magnitude to the NA study effect (latter is 3.0 points at week 52). Patients in the EU study were only allowed cholinesterase inhibitors, while the NA study also allowed memantine use. This difference in background AD medications may have contributed to the differences in ADAS-cog results between the two studies.
For the CDR-SB OC analysis, the low dose shows no benefit for visits up to week 52, similar to the NA study. The high dose shows consistent numerical benefit over placebo, and reaches maximum benefit at week 52, where the effect of 1.1 points is of similar magnitude to the NA study (latter is 0.7-1.1 at weeks 52 and 65).
The EU efficacy data are limited by the early termination of the study and the potential bias introduced at the early termination visits at weeks 65 and 78, but show numerical trends on CDR-SB that are of similar magnitude to the NA study.
Sensitivity Analysis for APOE4/4 Subgroup by Baseline MMSE
Analysis of efficacy based on baseline MMSE group was performed to evaluate for differences in drug response between mild and moderate patients. MMRM analyses were performed on the following MMSE categories: overall mild and moderate population (MMSE 16-26, inclusive); mild population (20-26, inclusive); and the milder population (22-26, inclusive). The data are shown in Figure 3 for ADAS-cog, CDR-SB, and DAD.
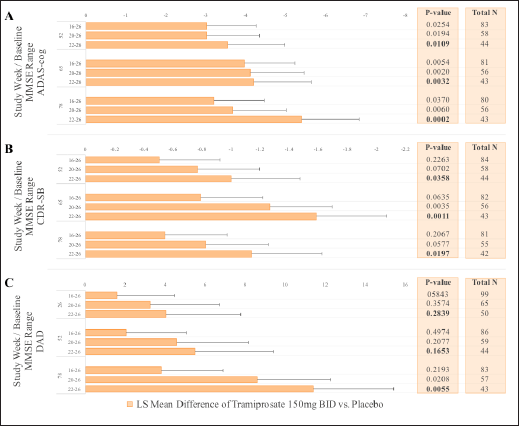
Figure 3. Effects of Tramiprosate 150 mg BID in APOE4/4 subgroup: Sensitivity analysis of effects on ADAS-cog and CDR-SB (Panels A, B) at weeks 52, 65 and 78. Effects on DAD are shown at weeks 26, 52 and 78 (Panel C). Sensitivity based on MMSE categories with increasing proportion of Mild Subjects (16-26, 20-26, and 22-26, NA Study). For ADAS-cog and CDR-SB outcomes, negative values in LS mean differences between drug and placebo indicate drug benefit; for DAD, positive values indicate drug benefit. Error bars are standard error of mean. Bolded (nominal) p-values are at week 78 endpoint.
These analyses suggest that APOE4/4 patients with mild AD (MMSE ≥20) may show greater benefit on the high dose of tramiprosate than those with lower MMSE at baseline. Additionally, patients with MMSE ≥22 (milder patients) showed the highest efficacy with a sustained cognitive improvement and less disability compared to placebo over the 78 weeks of the study.
Safety Analyses
Safety in the Combined NA and EU Studies
The nature of adverse events was similar between the two studies and between the three APOE4 subgroups. Therefore, the safety datasets were analyzed for the combined NA and EU studies (N= 1,052 and 973, respectively), and included a total of 2,025 unique AD patients who received at least 1 dose of study drug. The combined safety population (N= 2,025) included drug exposures up to 78 weeks.
In this safety population, the overall incidence of treatment emergent adverse events (TEAE) was slightly higher in the active dose arms than placebo (86-88% versus 81-82% in the APOE4 non-carriers and heterozygotes, respectively). In the APOE4/4 homozygous group the incidence was similar across the three dose arms, approximately 90%. The most common TEAEs in the overall safety population are shown in Table 5. The most common TEAEs with incidence more than double placebo rates were: nausea, vomiting, and decreased weight; and the majority were either mild or moderate in severity. The other TEAEs were similar in incidence between active arms and placebo.
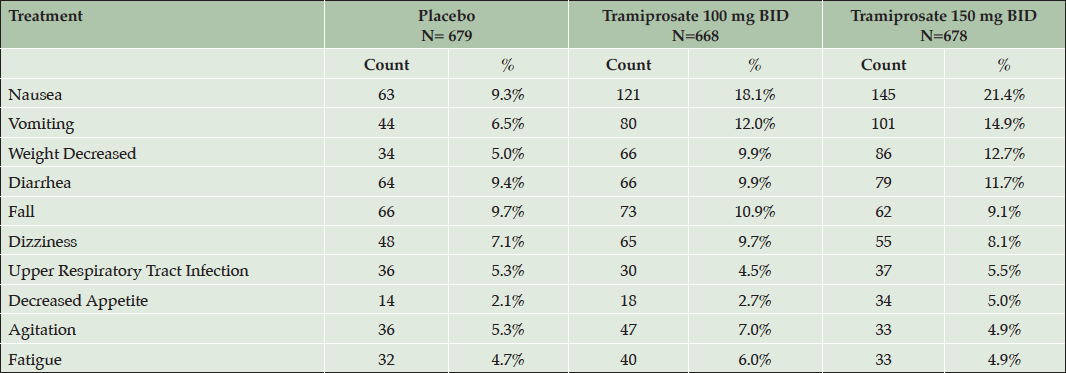
Table 5. Treatment emergent adverse events (TEAE) with incidence > 5% in any active dose arm in the safety population (combined safety population, all genotypes)
The overall rates of discontinuation due to AE in the APOE4 subgroups in the NA study were: 9% in homozygotes, 12% in heterozygotes and 13% in APOE4 non-carriers. In the EU study, the rates were: 14% in homozygotes, 9% in heterozygotes and 15% in APOE4 non-carriers.
In the overall safety population, the incidence of SAEs was similar across the three dose arms. In the APOE4/4 subgroup the incidence of SAEs was lower in the active dose arms than placebo. The most common SAEs that were double the placebo rate were syncope and pneumonia. The other SAEs are commonly reported in AD studies in the elderly population. Across both studies, there were a total of 14 deaths that were divided equally among the treatments arms: 5 in placebo, 5 in 100 mg BID, and 4 in 150 mg BID. The causes of death were typical of the elderly population in AD trials.
Central MRI Safety Assessments for ARIA
This MRI analysis included a total of 409 patients with scans at screening and week 78, or early termination, and an additional 17 subjects with MRI after study treatment only. MRI evaluations for ARIA revealed no cases of ARIA-E on active drug, and only one placebo APOE4/4 subject had possible ARIA-E. The distribution of subjects in each treatment arm by APOE4 genotype is presented in Table 6.
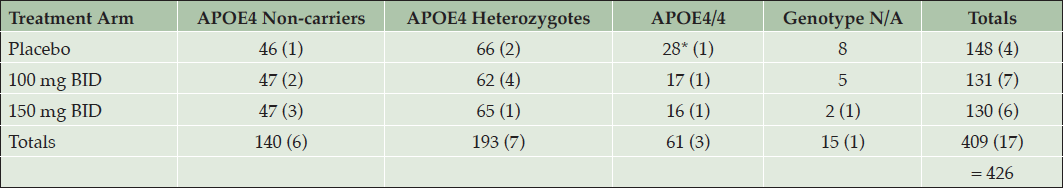
Table 6. MRI Safety Assessment for Vasogenic Edema: Distribution of Subjects across Treatment Arms and APOE4 Subgroups. In brackets are subjects who had only post-treatment MRI. * One subject with suspected ARIA-E.
Discussion
In AD patients clinical trials with amyloid-targeting agents regularly include APOE4 genotyping since APOE4 status influences age of onset and rate of decline, as well as susceptibility to drug-induced vasogenic edema and microhemorrhage. To our knowledge this is the first published analysis of drug efficacy in a clinical trial suggesting differential efficacy based on the number of APOE4 alleles. The findings suggest a gene dose-related benefit of tramiprosate with the largest drug-placebo difference in APOE4/4 homozygotes, intermediate benefit in APOE4 heterozygotes, and no benefit in APOE4 non-carriers.
The “APOE4 gene-dose effect” follows the well-established prevalence of β-amyloid positivity among the APOE4 homozygotes, heterozygotes and non-carrier subgroups in similar clinical trial populations, where study inclusion was based on a clinical diagnosis of AD. In the two solanezumab EXPEDITION trials in mild to moderate AD, the rate of positive amyloid PET (florbetapir) scans was 98% in homozygous group, 88% in heterozygous group, and 64% in non-carriers (11). In the two bapineuzumab studies (23), the rate of positive PET-PIB scans in non-carriers was similarly low at 62%. Since β-amyloid imaging was not available at the time of initiation of tramiprosate studies, the APOE4 genotype serves as a proxy for amyloid burden as well as accuracy of clinical AD diagnosis, which is very high in APOE4/4 homozygotes (>90%). Since tramiprosate is an amyloid-targeting agent, evaluating a population that is naturally enriched for amyloid pathology is critical to demonstrate drug efficacy. Another possible explanation for the gene-dose effect is that tramiprosate may have a direct effect on the APOE4 protein, a mechanism that is being investigated further in nonclinical studies. The latter potential mechanism may explain the difference in efficacy between the APOE4 homozygous and heterozygous subgroups.
The results of tramiprosate in the APOE4/4 homozygous subgroup suggest dose-dependent efficacy. Tramiprosate at the high 150 mg BID dose showed a beneficial cognitive effect that was nominally statistically significant and was supported by positive trends on functional outcomes. The low dose showed a lower numerical benefit on cognition but not on functional outcomes.
Drug effects in AD trials are considered clinically meaningful if they provide at least 25% benefit over placebo. The cognitive effect of the 150 mg BID dose in APOE4/4 AD patients corresponds to 40% benefit over placebo at week 78, and is thus clinically meaningful. This cognitive effect is also supported by positive trends on global function, which corresponds to 25% benefit on CDR-SB at the week 78 endpoint, as well as a numerically consistent effect on disability with 25% benefit on DAD. These effects were in patients who were already receiving current standard of care with either one or two AD symptomatic drugs, and thus have no other treatment options available at present. The additional tramiprosate efficacy could then provide a meaningful benefit in slowing the rate of decline and managing disease.
Based on the MMSE sensitivity analyses, tramiprosate efficacy appears to be greater and more sustained in mild AD patients (MMSE 20 and higher), as previously reported in several anti-amyloid antibody trials (24, 25). Clinical improvement appears to be largest in the mildest AD patients with baseline MMSE of 22 and higher, as reported in studies with other amyloid-targeting agents, scyllo-inositol and crenezumab (26, 27). In the mildest AD group ADAS-cog effects increase progressively from early visits to week 78, a pattern supportive of a potential disease modifying effect.
The above finding suggesting better efficacy on both cognition and function in mild AD patients is consistent with the mechanism of tramiprosate observed in preclinical studies. Tramiprosate inhibits aggregation of amyloid monomers into soluble oligomeric species that cause synaptic toxicity. Mild patients who have larger synaptic numbers and more synaptic integrity are therefore more likely to exhibit clinical benefit from an earlier protective effect of tramiprosate on synapses. The moderate AD subgroup appeared to show lesser effects on function but still showed potentially meaningful cognitive benefit.
The main limitation of the above efficacy analyses is that they are based on subgroup analyses from the NA study that did not achieve its primary objectives in the overall study population. The sample size of the APOE4/4 subgroup was also limited. Randomization was not stratified based on genotype and the genetically defined subgroups were not precisely comparable. Amyloid imaging was not available to determine the amyloid status of patients in the genetically-defined subgroups. Therefore, these findings require confirmation in prospectively defined studies in the APOE4/4 homozygous population.
From the safety perspective, tramiprosate showed a favorable safety profile based on placebo-controlled data in 2,025 patients in the combined Phase 3 studies. There was no evidence of dose-limiting vasogenic edema in the MRI subgroup. This could be an important advantage of tramiprosate, especially in APOE4 carriers who are at the highest risk of vasogenic edema with some anti-amyloid candidate agents (9, 28).
The most common adverse events were gastrointestinal: nausea, vomiting and weight loss, which were mostly mild or moderate in severity. This gastrointestinal tolerability issue is being addressed with the development of ALZ-801, a new tramiprosate formulation which is a valine prodrug of the active agent tramiprosate. ALZ-801 has shown substantially improved gastrointestinal tolerability as well as more consistent plasma levels of tramiprosate in Phase 1b studies in over 170 healthy elderly subjects (29). ALZ-801 at a dose that provides bioequivalent exposures to tramiprosate 150 mg BID will be used in future confirmatory studies focusing on the symptomatic APOE4/4 AD population.
Conclusions
APOE4/4 homozygotes constitute approximately 10-15% of all AD patients in memory clinics. Development of disease modifying drugs is challenging for this population due to their high burden of cortical and vascular amyloid pathology as well as their susceptibility to development of vasogenic edema and hemorrhage. Oral tramiprosate shows promising efficacy in symptomatic homozygous APOE4/4 AD patients at a dose that has a favorable safety profile with no observed ARIA-E. Future studies will focus on the homozygous APOE4/4 population with symptomatic AD. The use of the new prodrug ALZ-801, which provides more consistent plasma exposures and improved gastrointestinal tolerability, may further enhance efficacy and tolerability in these patients. Prospective confirmation of these promising efficacy findings in the homozygous APOE4/4 population with symptomatic AD, together with a favorable safety profile and the convenience of an oral formulation, could provide a major therapeutic advance for this genetically-defined population with a large unmet medical need.
Acknowledgements: We thank the investigators, their staff, and the individuals with AD who participated in the trials and their caregivers. We thank Dr. Sandra Saouaf and Christine Rathbun for editorial assistance.
Funding: Alzheon Inc. funded the above analyses and manuscript preparation. BELLUS Health (previously Neurochem), the original sponsor, had funded the tramiprosate Phase 3 studies.
Ethical standards: The study protocols were reviewed by ethics committees and complied with ICH standards for clinical trials and with all local requirements.
References
1. Roses AD. Apolipoprotein E alleles as risk factors in Alzheimer’s disease. Annu Rev Med. 1996;47:387-400.
2. Holtzman DM, Herz J, Bu G. Apolipoprotein E and apolipoprotein E receptors: normal biology and roles in Alzheimer disease. Cold Spring Harb Perspect Med. 2012;2:a006312.
3. Liu Y, Yu JT, Wang HF, et al. APOE genotype and neuroimaging markers of Alzheimer’s disease: systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2015;86:127-134.
4. Breitner JC, Wyse BW, Anthony JC, et al. APOE-epsilon4 count predicts age when prevalence of AD increases, then declines: the Cache County Study. Neurology. 1999;53:321-331.
5. Raber J, Huang Y, Ashford JW. ApoE genotype accounts for the vast majority of AD risk and AD pathology. Neurobiol Aging. 2004;25:641-650.
6. Petersen RC, Thomas RG, Grundman M, et al. Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med. 2005;352:2379-2388.
7. Lautner R, Palmqvist S, Mattsson N, et al. Apolipoprotein E genotype and the diagnostic accuracy of cerebrospinal fluid biomarkers for Alzheimer disease. JAMA Psychiatry. 2014;71:1183-1191.
8. Chalmers K, Wilcock GK, Love S. APOE epsilon 4 influences the pathological phenotype of Alzheimer’s disease by favouring cerebrovascular over parenchymal accumulation of A beta protein. Neuropathol Appl Neurobiol. 2003;29:231-238.
9. Sperling RA, Jack CR, Jr., Black SE, et al. Amyloid-related imaging abnormalities in amyloid-modifying therapeutic trials: recommendations from the Alzheimer’s Association Research Roundtable Workgroup. Alzheimers Dement. 2011;7:367-385.
10. Jansen WJ, Ossenkoppele R, Knol DL, et al. Prevalence of cerebral amyloid pathology in persons without dementia: a meta-analysis. JAMA. 2015;313:1924-1938.
11. Degenhardt EK, Witte MM, Case MG, et al. Florbetapir F18 PET Amyloid Neuroimaging and Characteristics in Patients With Mild and Moderate Alzheimer Dementia. Psychosomatics. 2016;57:208-216.
12. Gervais F, Paquette J, Morissette C, et al. Targeting soluble Abeta peptide with Tramiprosate for the treatment of brain amyloidosis. Neurobiol Aging. 2007;28:537-547.
13. Martineau E, de Guzman JM, Rodionova L, Kong X, Mayer PM, Aman AM. Investigation of the noncovalent interactions between anti-amyloid agents and amyloid beta peptides by ESI-MS. J Am Soc Mass Spectrom. 2010;21:1506-1514.
14. Aisen PS, Saumier D, Briand R, et al. A Phase II study targeting amyloid-beta with 3APS in mild-to-moderate Alzheimer disease. Neurology. 2006;67:1757-1763.
15. Aisen PS, Gauthier S, Ferris SH, et al. Tramiprosate in mild-to-moderate Alzheimer’s disease – a randomized, double-blind, placebo-controlled, multi-centre study (the Alphase Study). Arch Med Sci. 2011;7:102-111.
16. Mohs RC, Cohen L. Alzheimer’s Disease Assessment Scale (ADAS). Psychopharmacol Bull. 1988;24:627-628.
17. Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43:2412-2414.
18. Gelinas I, Gauthier L, McIntyre M, Gauthier S. Development of a functional measure for persons with Alzheimer’s disease: the disability assessment for dementia. Am J Occup Ther. 1999;53:471-481.
19. Folstein MF, Folstein SE, McHugh PR. «Mini-mental state». A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189-198.
20. Cummings JL. The Neuropsychiatric Inventory: assessing psychopathology in dementia patients. Neurology. 1997;48:S10-16.
21. Gauthier S, Aisen PS, Ferris SH, et al. Effect of tramiprosate in patients with mild-to-moderate Alzheimer’s disease: exploratory analyses of the MRI sub-group of the Alphase study. J Nutr Health Aging. 2009;13:550-557.
22. Blacker D, Haines JL, Rodes L, et al. ApoE-4 and age at onset of Alzheimer’s disease: the NIMH genetics initiative. Neurology. 1997;48:139-147.
23. Salloway S, Sperling R, Fox NC, et al. Two phase 3 trials of bapineuzumab in mild-to-moderate Alzheimer’s disease. N Engl J Med. 2014;370:322-333.
24. Doody RS, Farlow M, Aisen PS. Phase 3 trials of solanezumab and bapineuzumab for Alzheimer’s disease. N Engl J Med. 2014;370:1460.
25. Lasser R, Ostrowitzki S, Scheltens P, et al. Efficacy and safety of gantenerumab in prodromal Alzheimer’s disease: Results from scarlet road—a global, multicenter trial. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association. 2015;11:P331-P332.
26. Cummings J, Cho W, Ward M, et al. A randomized, double-blind, placebo-controlled phase 2 study to evaluate the efficacy and safety of crenezumab in patients with mild to moderate Alzheimer’s disease. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association. 2014;10:P275.
27. Salloway S, Sperling R, Keren R, et al. A phase 2 randomized trial of ELND005, scyllo-inositol, in mild to moderate Alzheimer disease. Neurology. 2011;77:1253-1262.
28. Sevigny J, Chiao P, Bussiere T, et al. The antibody aducanumab reduces Abeta plaques in Alzheimer’s disease. Nature. 2016;537:50-56.
29. Hey JA, Abushakra S, Power A, Yu JY, Versavel M, Tolar M. Phase 1 development of ALZ-801, a novel beta amyloid anti-aggregation prodrug of tramiprosate with improved drug properties, supporting bridging to the phase 3 program. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association. 2016;in press.
